Video: Nephron-Sparing Surgery Across the UK
Nephron-sparing surgery across a nation – outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit
Objective
To determine the scope and outcomes of nephron-sparing surgery (NSS), i.e. partial nephrectomy, across the UK and in so doing set a realistic benchmark and identify fresh contemporary challenges in NSS.
Patients and Methods
Results
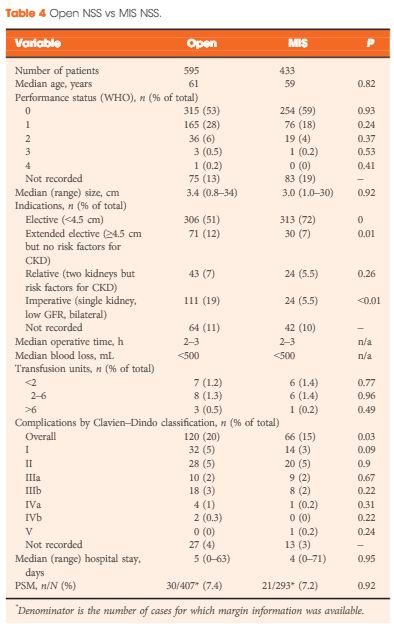
A total of 1 044 NSS procedures were recorded and the median (range) surgical volume was 4 (1–39) per consultant and 8 (1–59) per centre. In all, 36 surgeons and 10 centres reported on only one NSS. The indications for NSS were: elective with a tumour of ≤4.5 cm in 59%, elective with a tumour of >4.5 cm in 10%, relative in 7%, imperative in 12%, Von Hippel–Lindau in 1%, and unknown in 11%. The median (range) tumour size was 3.4 (0.8–30) cm. The technique used was minimally invasive surgery in 42%, open in 58%, with conversions in 4%. The histology results were: malignant in 80%, benign in 18%, and unknown in 2%. In patients aged <40 years 36% (36/101) had benign histology vs 17% (151/874) of those aged ≥40 years (P < 0.01). In patients with tumours of <2.5 cm 29% (69/238) had benign histology vs 14% (57/410) with tumours of 2.5–4 cm vs 8% (16/194) with tumours of ≥4 cm (P = 0.02). In patients aged <40 years with of tumours of <2.5 cm 44% (15/34) were benign. The 30-day mortality was 0.1% (1/1 044). There were major complications (Clavien–Dindo grade of ≥IIIa) in 5% (53/1 044). There was an increased risk of complications after extended elective NSS of 19% (19/101) vs elective at 12% (76/621) (relative risk [RR] 1.54; P < 0.01). Margins were recorded in 68% (709/1 044) of the patients, with positive margins identified in 7% (51/709). Positive surgical margins after NSS for pathological T3 (pT3) tumours were found in 47.8% (11/23) vs 6.1% (32/523) for pT1a, tumours (RR 5.61; P < 0.01). In all, 14% (894/6 042) of the patients underwent surgery for T1a tumours: 55% (488/894) by NSS, 42% (377/894) by radical nephrectomy (RN), and in 3% (29/894) the procedure used was unknown. Major complications after occurred in 4.9% (24/488) of NSS vs 1.3% (5/377) of RN (P < 0.01). Limitations included poor reporting of renal function data and no data on tumour complexity.
Conclusions
In its first year, mandatory national reporting has provided several challenging contemporary insights into NSS.