Editorial: Reach for the sky – tissue engineering in urology
The work of Verdi et al. [1] published in this issue, shows the continuing quest to find a cellular substrate suitable for producing a tissue engineered replacement for detrusor smooth muscle. This study has identified the regenerative ability of endometrium and with the use of myogenic culture media has sought to differentiate stem cells of endometrial origin to produce the desired smooth muscle cells. The ultimate objective is to produce a functional organ replacement that improves on the current methods of tissue replacement. The current standard for bladder replacement is bowel, both large and small, in various eponymous configurations. All cystoplasties have the potential for long-term consequences including metabolic derangement, UTI, stone formation and mucus secretion [2]. They also suffer the limitation that they will not contract, thus between 10% and 75% will need to self-catheterise. However, many patients do very well after reconstruction with bowel, thus it is important that any substrate designed to replace the current standard matches and improves on that which bowel can offer.
The complex interactions required to achieve a functional bladder replacement are discussed by many authors and include that with urothelium [3], nerve growth and angiogenesis. Despite considerable ingenuity only some of these concerns are solved by previous approaches that have, for example, seeded urothelium onto a vascularised, de-epithelialised flap [4]. The attempt to generate a true composite bladder using cultured urothelium and muscle generated from their native source has been through animal and some clinical exposure but thus far have not gained widespread acceptance and usage – suggesting continued limitations [5, 6].
The pluripotent stem cell approach is attractive, as tissue can be generated from a source distant from the organ needing regeneration, thus bypassing any inherent disease process. The creation of an environment that pushes cellular differentiation along the desired path is the premise by which this works.
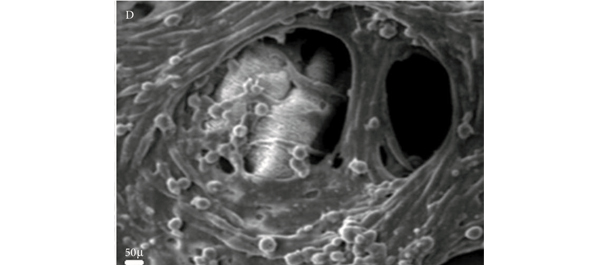
The authors of the current work [1] have analysed the population of generated cells using immunohistochemistry, scanning electron microscopy, gene expression analysis and Western blotting. From this we can learn that the cells are reproducible, viable and appear to exhibit characteristics of the desired smooth muscle cell. That said, all of the current models lack the most desirable of goals – that of controlled, functional similarity to the native bladder. The authors of this paper make the inference that the presence of α-smooth muscle actin suggests that the cells will be contractile. The experiments presented here may imply that but do not confirm it.
The field of tissue engineering remains exciting and authors such as these and others are to be congratulated on continuing to seek innovative approaches to solve a complex problem. The goal is organ replacement and clinical application. Each step along the path to that achievement is valuable but researchers working in the field need to ensure that they remain true to that aim. Cellular markers are only one part of a picture and future work must link them with function in novel cell populations. Once linked with function the means by which function is then controlled becomes important. Before we can safely apply this technology to patients, we must be clear about the functional abilities and limitations of the tissue created, this should be by evidence and not implication. Whilst those undertaking the research convey an optimistic view, the ability to understand the long-term viability and cellular stability remain significant unknowns.
Dan Wood
Adolescent and Paediatric Urology, University College London Hospitals, London, UK
References
- Verdi J, Shoae-Hassani A, Sharif S, Seifalian AM, Mortazavi-Tabatabaei SA, Rezaie S. Endometrial stem cell differentiation into smooth muscle cell: a novel approach for bladder tissue engineering in women. BJU Int 2013; 112: 854–863
- Biers SM, Venn SN, Greenwell TJ. The past, present and future of augmentation cystoplasty. BJU Int 2012; 109: 1280–1293
- Cross WR, Eardley I, Leese HJ, Southgate J. A biomimetic tissue from cultured normal human urothelial cells: analysis of physiological function. Am J Physiol Renal Physiol 2005; 289: F459–468
- Fraser M, Thomas DF, Pitt E, Harnden P, Trejdosiewicz LK, Southgate J. A surgical model of composite cystoplasty with cultured urothelial cells: a controlled study of gross outcome and urothelial phenotype. BJU Int 2004; 93: 609–616
- Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 1999; 17: 149–155
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006; 367: 1241–1246