Article of the month: Effect of timing of an immediate instillation of mitomycin C after TUR in 941 patients with NMIBC
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
The effect of timing of an immediate instillation of mitomycin C after transurethral resection in 941 patients with non-muscle-invasive bladder cancer
Abstract
Objective
To investigate whether the timing of an immediate instillation of mitomycin C (on the day of transurethral resection of bladder tumour [TURBT] or 1 day later) has an impact on time to recurrence of non‐muscle‐invasive bladder cancer (NMIBC).
Patients and Methods
All patients with NMIBC who were enrolled in a prospective trial between 1998 and 2003, and treated with an early mitomycin C instillation (on the day of TURBT or 1 day later), were selected. Statistical analysis was performed with Kaplan–Meier curves and multivariable Cox regression.
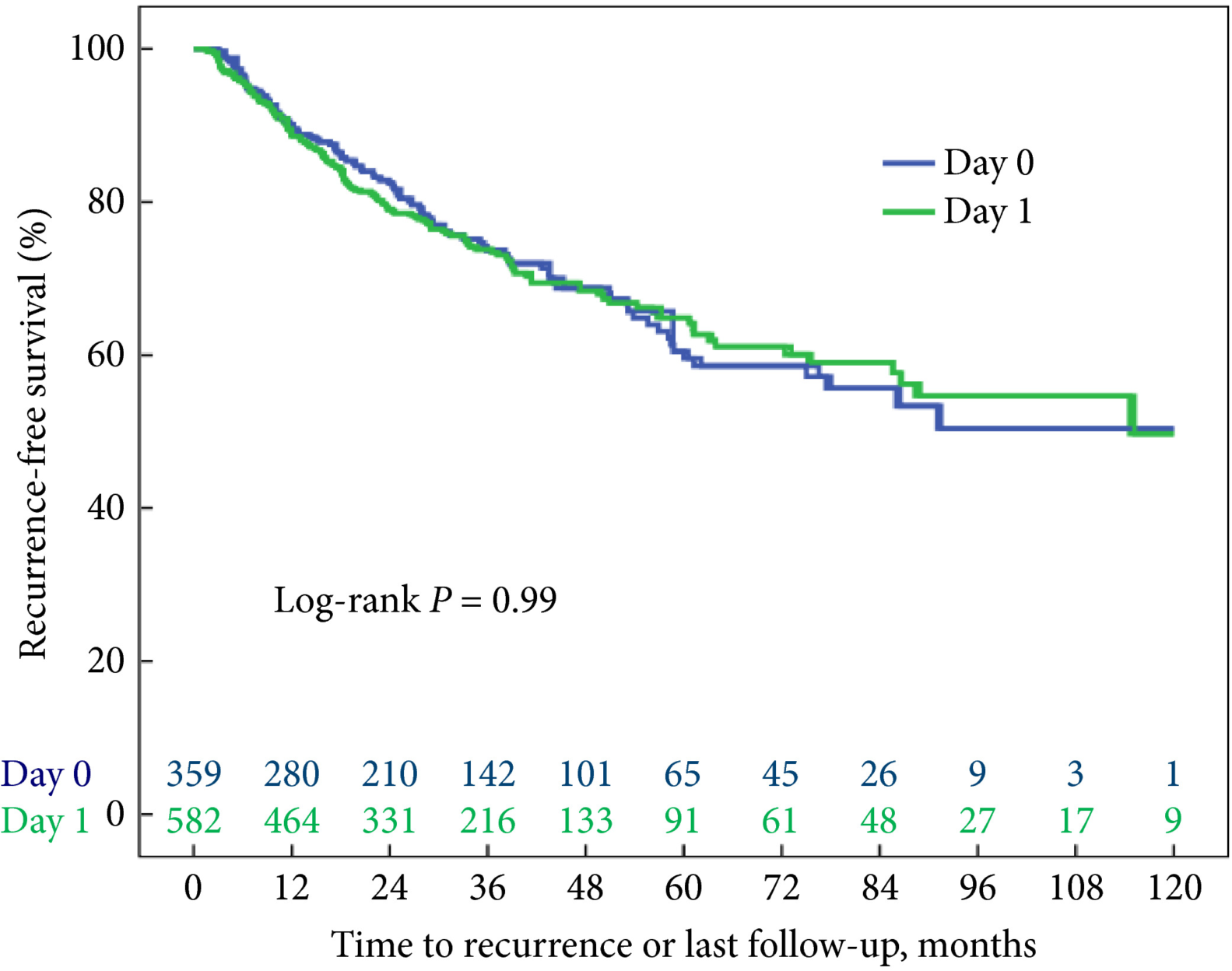
Fig. 1 Kaplan–Meier analysis showing time to recurrence for patients treated with an immediate instillation of MMC on the day of TURBT (Day‐0 group) or 1 day after (Day‐1 group).
Results
Administering an instillation of mitomycin C on the day of TURBT or 1 day later did not show a statistically significant difference in time to recurrence in a univariable model (log‐rank P = 0.99). After correcting for the number of scheduled adjuvant instillations, no statistically significant difference could be detected either: hazard ratio 1.05 (95% confidence interval 0.81–1.35, P = 0.74).
Conclusion
These data do not support the hypothesis that a very early instillation (on the day of TURBT) of mitomycin C decreases the risk of recurrence as compared with an early instillation (1 day after TURBT).