A further re‐engineering of the prostatic stent (temporary implantable nitinol device [TIND®, Medi‐Tate Ltd., Israel]) is reported by Porpiglia et al. 1 in this edition of the BJUI. Enthusiasm for novel ‘minimally invasive’ treatments for BPH/LUTs has surged recently, with new options supported by strong data becoming available 2, 3. That interest reflects the continuing desire of our patients to seek alternatives to the standard drug or surgical therapies.
The success of UroLift® (Neotract, Pleasanton, CA, USA) in satisfying the rigour of the regulatory bodies (especially the Food and Drug Administration [FDA] in the USA and National Institute for Health and Care Excellence [NICE] in the UK) and the recent merger/acquisition of Neotract by Teleflex Inc. for a sum potentially exceeding 1 billion dollars, indicates the rewards available for a novel treatment with convincing data. Not surprisingly, this has stimulated innovators, so there is a veritable tsunami of new treatments approaching us. The rewards are certainly encouraging, but it is worth remembering that the difficulties and cost of reaching market and the point of sale are now enormous.
The TIND stent is implanted endoscopically and then gradually distends to cause three linear pressure necrosis effects within the prostatic urethra and bladder neck tissues, before it is removed at approximately 5–7 days by a second, minor and office‐based procedure.
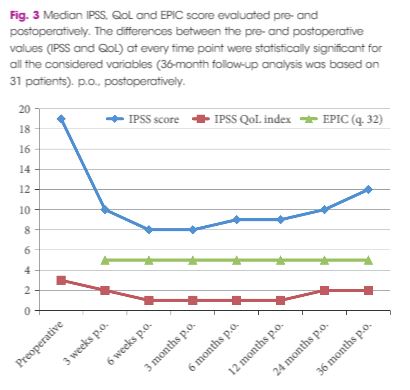
However, this report is simply a repeat of the initial case series from 2015 4, just with follow‐up to 36 months. An initial report is legitimate in order to show primarily safety and with effectiveness as a secondary outcome measure. I see limited value in a follow‐up report of a device that has already been superseded by a second version, which is currently being assessed in the more rigorous studies that we, our patients, and regulators require.
Even so, there are some important messages and some inconsistencies. Porpiglia et al. 1 are, firstly, to be commended for successfully following their patients out to 3 years with essentially complete data sets. That is remarkable. Although the TIND seems safe, there was one serious complication (a prostatic abscess associated with arrhythmia, sepsis and hyperglycaemia, which required 10 days of inpatient care). About 10% of men restarted medical therapy, but are not ‘failures’, as failures are defined only as those requiring surgical treatment. The prostate size in this small cohort is also surprisingly small compared to the initial studies of other novel devices (29.5 vs 50 mL for UroLift in the initial report) 5. It is also surprising that even though all were on α‐blockers and a substantial proportion were taking 5α‐reductase inhibitors that there is no sexual or especially ejaculatory dysfunction in any of the 19 sexually active men before or after TIND implantation. They appear to have surprisingly resilient ejaculatory function and one wonders why or how? The population studied may therefore not be representative of men we meet in our clinics. Nevertheless, the safety and effectiveness of the original TIND stent do seem reasonable and many of us will await the definitive ‘pivotal’ randomised controlled studies of the successor device with great interest.
For the TIND to find a place in our range of treatments, we urologists, our patients, and regulators of healthcare, now need to see proper randomised controlled trials, preferably incorporating a sham arm and eventually comparisons with TURP. Ideally, the TIND should also be compared to UroLift and to steam injection (Rezūm®; NxThera, Inc., Maple Grove, MN, USA). But who will pay for these studies, even if the regulators insist on them? Funding the required studies is now a major financial challenge to any new device in development. Those studies cost more than we think. A survey of >200 MedTech companies in 2010 suggested device development costs up to 100 million dollars (the majority spent on FDA‐dependent or related activities) but for UroLift total development spend was nearly 240 million. Unfortunately, even innovative and potentially useful devices will struggle to overcome the double jeopardy of regulatory hurdles and obtaining the finance required to clear them. I wish the developers of TIND well and hope there is adequate funding. Today, developers need deep pockets to perform the proper studies correctly.
Tom McNicholas
Pinehill Hospital, Hitchin, Herts, UK
References
- 1 Porpiglia F, Fiori C, Bertolo R et al. 3‐Year follow‐up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int2018; 122: 106–12
- 2 Roehrborn CG, Barkin J, Gange SN et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol 2017; 24: 8802–13
- 3 McVary KT, Roehrborn CG. Three‐year outcomes of the prospective, randomized controlled Rezūm System study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology 2018; 111: 1–9
- 4 Porpiglia F, Fiori C, Bertolo R, Garrou D, Cattaneo G, Amparore D. Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at 1 year of follow‐up. BJU Int 2015; 116: 278–87
- 5 Woo HH, Chin PT, McNicholas TA et al. Safety and feasibility of the prostatic urethral lift: a novel, minimally invasive treatment for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). BJU Int 2011; 108: 82–8