Editorial: Systematic transperineal and MRI‐targeted biopsies: the resolution of uncertainty
The paper published in this issue of the BJUI titled ‘Multicentre evaluation of magnetic resonance imaging supported transperineal biopsy in biopsy‐naïve men with suspicion of prostate cancer’ is timely and helps to resolve some of the uncertainty inherent within the diagnostic pathway 1.
The publication of the PROstate MRI Imaging Study (PROMIS) study, although demonstrating that 25% of patients might avoid prostate biopsy with a normal MRI (Prostate Imaging Reporting and Data System [PI‐RADS] 1–2) and that MRI could identify 90% of patients with high‐risk disease (PI‐RADS 5), did not resolve the issue of what to do with equivocal PI‐RADS 3 scans, uncertainty remained 2. The recent publication of the PRECISION trial (Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not?) has only contributed to the uncertainty of systematic TRUS biopsy and has shown that targeted biopsies resolve the issue for <50% of the patients overall and only 12% of those with PI‐RADS 3 lesions had a diagnosis of cancer on targeted biopsy only 3. The study has shown that in the face of an identifiable lesion a MRI‐targeted biopsy is non‐inferior to a blind systematic TRUS biopsy, which was positive in only 28% and implies that a systematic biopsy may be unnecessary, so where does that leave us? The uncertainty within MRI remains at the PI‐RADS 3 level, and particularly with a TRUS biopsy that is not a systematic biopsy of the peripheral zone. The authors of the paper highlighted in this issue of the BJUI 1 help to resolve the issue because they describe a more systematic biopsy.
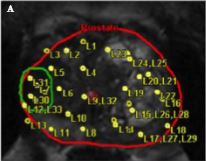
The transperineal (TP) biopsy approach for systematic and targeted biopsy they use is that which was adopted by the Ginsburg Study Group on Enhanced Prostate Diagnostics 4. It is a systematic biopsy that preferentially targets the peripheral zone in a sectoral fashion. It avoids the oversampling inherent in template‐mapping biopsy and the under‐sampling of the non‐systematic transrectal biopsy. Their paper evaluates the combination of an MRI‐targeted biopsy with a systematic TP biopsy. It confirms, as suggested by the PROMIS study, that patients with PI‐RADS 1 or 2 prostates on MRI with a low PSA density <0.1 ng/mL/mL could safely avoid biopsy, based upon a negative predictive value of 0.91 on systematic biopsy. However, in 418 patients with PI‐RADS 4–5 lesions, it was the combination of a targeted and systematic TP biopsy that achieved an overall cancer detection rate of 71%, but that MRI‐targeted biopsies alone had a detection rate of 59% vs 61% for systematic TP biopsies. In the PI‐RAD 3 equivocal group the combined biopsy identified 30% with Gleason score 7–10, whereas targeted biopsy only was positive in 21% vs 27% with systematic biopsies.
The message is clear.
An appropriate systematic biopsy targeted to the peripheral zone remains an essential component of prostate diagnosis even in the MRI era, as indeed it did before MRI was available. In the pre‐MRI days, about one‐third of patients that had negative TRUS biopsies had cancer on TP biopsies and a third of those thought suitable for AS on TRUS biopsy had more significant disease. I suspect in the modern era that figure remains unchanged for those with PI‐RADS 1, 2 or 3, particularly with a PSA density >0.15 ng/mL/mL. As urologists we have always been criticised for over diagnosing and over treating prostate cancer but I suspect that the more heinous crime is that of under treatment of significant disease, it is the very reason why I started doing TP biopsies, to resolve uncertainty. I consider that MRI, for all its benefits in the diagnostic algorithm, cannot yet resolve that uncertainty.
Probably the only patients that merit a target‐only biopsy are those with the high‐PSA, large‐volume disease, easily visible on MRI and usually palpable. Prostate biopsy can be avoided or at least deferred in the PI‐RADS 1–2 group with low PSA density; the rest should be offered a systematic biopsy along with a targeted biopsy. This may be less important in those proceeding to whole gland treatment or surgical extirpation but remains essential in those considering active surveillance, brachytherapy, or any one of the myriad of unproven focal treatments becoming available. The authors should be congratulated for bringing some certainty to uncertainty.
- 1 Hansen NL, Barrett T, Kesch C et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy‐naïve men with suspicion of prostate cancer. BJU Int 2018; 122: 40–9
- 2 Ahmed HU, El‐Shater Bosaily A, Brown LC et al. Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22
- 3 Kasivisvanathan V, Ranniko A, Borghi M et al. MRI‐targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med 2018. https://doi.org/10.1056/NEJMoa1801993 [Epub ahead of print]
- 4 Kuru TH, Wadhwa K, Chang RT et al. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int 2013; 112: 568–77