Article of the week: Patient‐reported outcomes in a phase 2 study comparing atezolizumab alone or with bevacizumab vs sunitinib in previously untreated metastatic renal cell carcinoma
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
If you only have time to read one article this week, we recommend this one.
Patient‐reported outcomes in a phase 2 study comparing atezolizumab alone or with bevacizumab vs sunitinib in previously untreated metastatic renal cell carcinoma
Sumanta K. Pal*, David F. McDermott†, Michael B. Atkins‡, Bernard Escudier§, Brian I. Rini¶, Robert J. Motzer**, Lawrence Fong††, Richard W. Joseph‡‡, Stephane Oudard§§, Alain Ravaud¶¶, Sergio Bracarda***, Cristina Suárez†††, Elaine T. Lam‡‡‡, Toni K. Choueiri§§§, Beiying Ding¶¶¶, Caroleen Quach¶¶¶, Kenji Hashimoto****, Christina Schiff¶¶¶, Elisabeth Piault-Louis¶¶¶ and Thomas Powles††††
*Department of Medical Oncology and Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, †Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, ‡Georgetown Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC, USA, §Gustave Roussy, Villejuif, France, ¶Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH, **Memorial Sloan Kettering Cancer Center, New York, NY, ††School of Medicine, University of California, San Francisco, San Francisco, CA, ‡‡Mayo Clinic Hospital, Jacksonville, FL, USA, §§Department of Medical Oncology, Georges Pompidou Hospital, Paris Descartes University, Paris, ¶¶CHU Hôpitaux de Bordeaux, Hôpital Saint-André, Bordeaux, France, ***Azienda Ospedaliera S. Maria, Terni, Italy, †††Vall d’Hebron University Hospital and Institute of Oncology, Barcelona, Spain, ‡‡‡Anschutz Medical Campus, University of Colorado, Aurora, CO, §§§Dana-Farber Cancer Institute, Boston, MA, ¶¶¶Genentech, Inc., South San Francisco, CA, USA,****Roche Products Ltd, Welwyn Garden City, and ††††Barts Cancer Institute, Royal Free Hospital, Queen Mary University of London, London, UK
Abstract
Objective
To evaluate patient‐reported outcome (PRO) data from the IMmotion150 study. The phase 2 IMmotion150 study showed improved progression‐free survival with atezolizumab plus bevacizumab vs sunitinib in patients with programmed death‐ligand 1 (PD‐L1)+ tumours and suggested activity of atezolizumab monotherapy in previously untreated metastatic renal cell carcinoma (mRCC).
Patients and methods
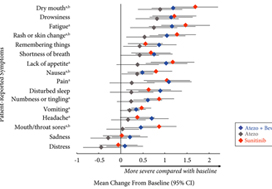
Patients with previously untreated mRCC were randomised to atezolizumab 1200 mg intravenously (i.v.) every 3 weeks (n = 103), the atezolizumab regimen plus bevacizumab 15 mg/kg i.v. every 3 weeks (n = 101), or sunitinib 50 mg orally daily (4 weeks on, 2 weeks off; n = 101). The MD Anderson Symptom Inventory (MDASI) and Brief Fatigue Inventory (BFI) were administered on days 1 and 22 of each 6‐week cycle. Time to deterioration (TTD), change from baseline in MDASI core and RCC symptom severity, interference with daily life, and BFI fatigue severity and interference scores were reported for all comers. The TTD was the first ≥2‐point score increase over baseline. Absolute effect size ≥0.2 suggested a clinically important difference with checkpoint inhibitor therapy vs sunitinib.
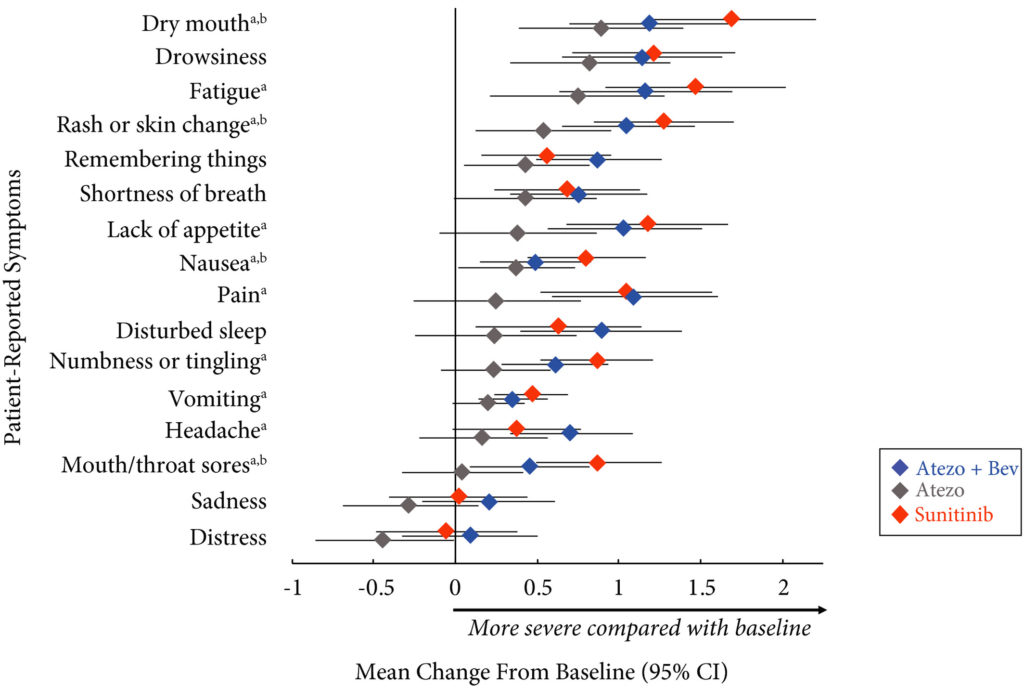
Results
Completion rates were >90% at baseline and ≥80% at most visits. Delayed TTD in core and RCC symptoms, symptom interference, fatigue, and fatigue‐related interference was observed with atezolizumab (both alone and in combination) vs sunitinib. Improved TTD (hazard ratio [HR], 95% confidence interval [CI]) was more pronounced with atezolizumab monotherapy: core symptoms, 0.39 (0.22–0.71); RCC symptoms, 0.22 (0.12–0.41); and symptom interference, 0.36 (0.22–0.58). Change from baseline by visit, evaluated by the MDASI, also showed a trend favouring atezolizumab monotherapy vs sunitinib. Small sample sizes may have limited the ability to draw definitive conclusions.
Conclusion
PROs suggested that atezolizumab alone or with bevacizumab maintained daily function compared with sunitinib. Notably, symptoms were least severe with atezolizumab alone vs sunitinib (IMmotion150; ClinicalTrials.gov Identifier: NCT01984242).