Article of the Week: Prostate Health Index density improves detection of clinically significant prostate cancer
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video discussing the paper.
If you only have time to read one article this week, it should be this one.
Prostate Health Index density improves detection of clinically significant prostate cancer
Abstract
Objectives
To explore the utility of Prostate Health Index (PHI) density for the detection of clinically significant prostate cancer (PCa) in a contemporary cohort of men presenting for diagnostic evaluation of PCa.
Patients and Methods
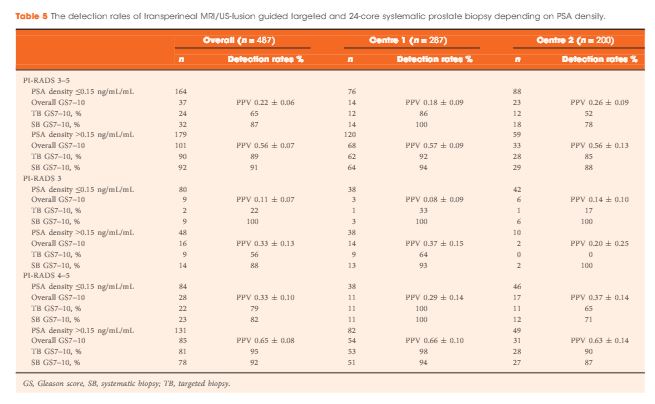
The study cohort included patients with elevated prostate-specific antigen (PSA; >2 ng/mL) and negative digital rectal examination who underwent PHI testing and prostate biopsy at our institution in 2015. Serum markers were prospectively measured per standard clinical pathway. PHI was calculated as ([{−2}proPSA/free PSA] × [PSA]½), and density calculations were performed using prostate volume as determined by transrectal ultrasonography. Logistic regression was used to assess the ability of serum markers to predict clinically significant PCa, defined as any Gleason score ≥7 cancer or Gleason score 6 cancer in >2 cores or >50% of any positive core.
Results
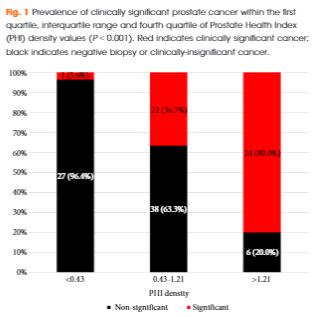
Of 118 men with PHI testing who underwent biopsy, 47 (39.8%) were found to have clinically significant PCa on biopsy. The median (interquartile range [IQR]) PHI density was 0.70 (0.43–1.21), and was 0.53 (0.36–0.75) in men with negative biopsy or clinically insignificant PCa and 1.21 (0.74–1.88) in men with clinically significant PCa (P < 0.001). Clinically significant PCa was detected in 3.6% of men in the first quartile of PHI density (<0.43), 36.7% of men in the IQR of PHI density (0.43–1.21), and 80.0% of men with PHI density >1.21 (P < 0.001). Using a threshold of 0.43, PHI density was 97.9% sensitive and 38.0% specific for clinically significant PCa, and 100% sensitive for Gleason score ≥7 disease. Compared with PSA (area under the curve [AUC] 0.52), PSA density (AUC 0.70), %free PSA (AUC 0.75), the product of %free PSA and prostate volume (AUC 0.79), and PHI (AUC 0.76), PHI density had the highest discriminative ability for clinically significant PCa (AUC 0.84).
Conclusions
Based on the present prospective single-centre experience, PHI density could be used to avoid 38% of unnecessary biopsies, while failing to detect only 2% of clinically significant cancers.