Hydronephrosis of Pregnancy in an Ectopic Pelvic Kidney
We present a case of a woman with an ectopic pelvic kidney and symptomatic hydronephrosis who failed conservative treatment and required ureteric stent placement.
Authors: Toepfer, Nicholas; Parikh, Ankur; Bhangdia, Darshan
Corresponding Author: Toepfer, Nicholas J
Introduction
Hydronephrosis during pregnancy is common and has a reported occurrence between 43% and 100% (1). Both mechanical and hormonal changes during pregnancy are thought to contribute to its etiology, but evidence suggests it is mainly due to compression of the ureters between the pregnant uterus and the linea terminalis (2,3). It has been postulated that this phenomenon does not affect women whose ureters do not cross the pelvic brim. The incidence of an ectopic pelvic kidney is approximately 1 in 2200 to 3000 patients (4,5). Ectopic kidneys occur due to failure of the kidney to ascend during weeks 6-9 of fetal development. Although ectopic pelvic kidneys are relatively common, only one case of hydronephrosis during pregnancy in a pelvic kidney is described in the literature (6). This supports the notion that hydronephrosis during pregnancy in pelvic kidneys is exceedingly rare. We present a case of a woman with an ectopic pelvic kidney and symptomatic hydronephrosis who failed conservative treatment and required ureteric stent placement.
Case Presentation
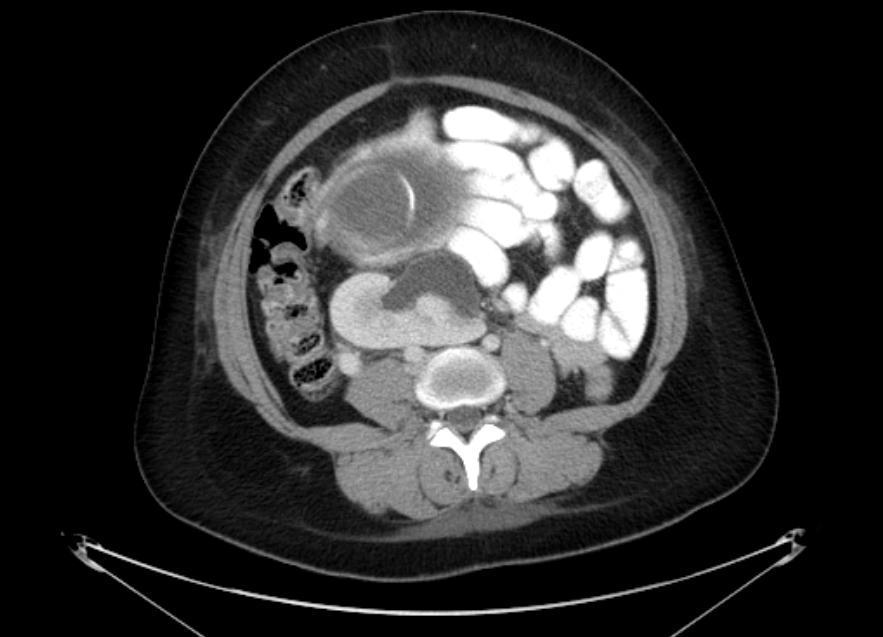
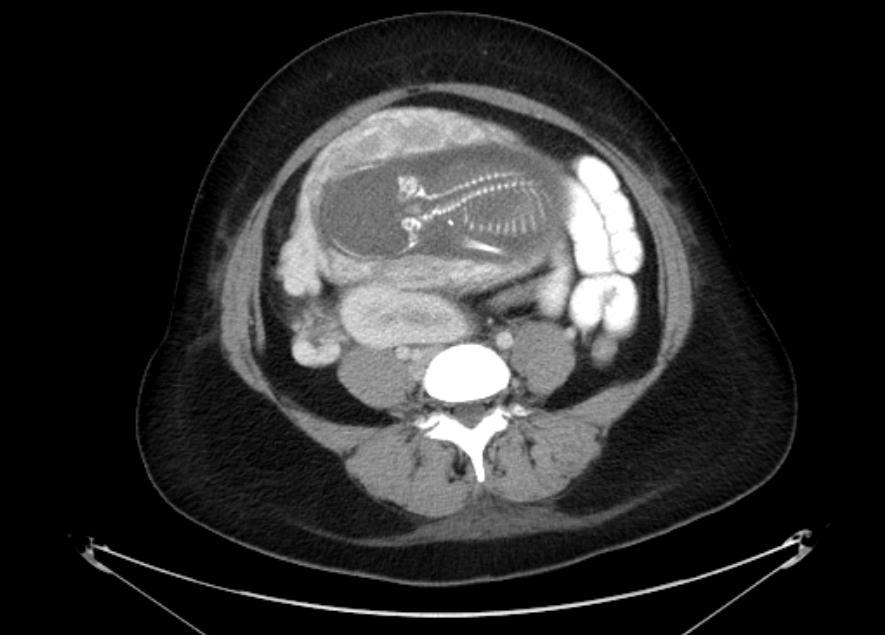
A 31 year old pregnant female at 22 weeks gestation presented with complaints of sharp right lower quadrant pain, nausea, and vomiting. She denied any lower urinary tract symptoms. On physical examination, she was afebrile and haemodynamically stable. The gravid abdomen was soft, non-tender and no pain could be elicited on palpation. Her urinalysis was negative for leukocyte esterase and nitrite. She demonstrated a leukocytosis of 12.7 K/µL with 88% segmented neutrophils and 7% lymphocytes. A CT scan of the abdomen and pelvis with intravenous contrast was performed for suspected acute appendicitis. The CT scan showed an ectopic right kidney located in the pelvis with mild hydronephrosis of an anteriorly oriented collecting system (Figure 1a-b). The ectopic kidney was malrotated, medially deviated, and located anterior to the iliac bifurcation and immediately posterior to the gravid uterus. The left kidney was unremarkable and located in the orthotopic location in the left retroperitoneum. The patient’s severe right lower quadrant pain persisted despite intravenous analgesia. The patient was transferred to a tertiary medical center for further care.
The patient was counseled on the options regarding symptomatic hydronephrosis during pregnancy and she opted for conservative management with intravenous and oral analgesia. She continued to experience intractable abdominal pain and after 48 hours of medical management, she decided to undergo a cystoscopy with retrograde ureteric stent placement.
At operation, there were no abnormal findings on cystoscopy. A retrograde pyelogram was performed. The ureter had no evidence of filling defects as it coursed to a midline kidney located at the level of the L4 vertebral body. An 18 cm long 4.7 Fr diameter stent was placed and the location was confirmed using fluoroscopy. Postoperatively, the patient’s pain completely resolved but she did report mild bladder irritation, frequency and urgency from the ureteric stent.
The patient underwent exchange of her stent 12 weeks later. She eventually underwent a caesarean section for a breech presentation at 39 weeks. This was completed without complications and the newborn baby was without any identifiable abnormalities. The ureteric stent was removed 12 days later and the patient remained asymptomatic afterwards. A renal ultrasound one month after stent removal demonstrated resolution of the hydronephrosis (Figure 2).
Discussion
Hydronephrosis can manifest at different stages during pregnancy. A large prospective study found hydronephrosis present in 15%, 20% and 50% of women during the first, second, and third trimesters respectively.1 The development of dilatation at various stages of pregnancy may represent different etiologies of hydronephrosis in pregnancy including mechanical and hormonal effects.
The 15% incidence of ureteric dilation on ultrasound during the first trimester occurs before the uterus reaches the pelvic brim. This supports a non-mechanical etiology for the development of hydronephrosis. Increased levels of progesterone during pregnancy have been hypothesized to promote ureteric dilatation and inhibit peristalsis which causes subsequent dilation of the upper urinary tract (1,7). In addition, progesterone has been shown to slow the rate of disappearance of hydroureter in postpartum women (8).
While hormonal changes may contribute to hydronephrosis of pregnancy, the most plausible etiologic factor is mechanical obstruction of the ureter by the gravid uterus. Studies have shown normal ureteral contractile pressures in pregnant women suggesting that hormonal induced atony is not the primary factor in ureteric dilation (2). Additionally, women whose ureters do not cross the pelvic brim, such as those associated with ectopic and pelvic kidneys or non-orthotopic urinary diversion, have been shown not to develop ureteric dilation of pregnancy (9-11). Despite this evidence, our patient demonstrates that women with pelvic kidneys can develop hydronephrosis during pregnancy.
Pelvic kidneys present challenges in treatment for a variety of reasons including tortuosity of the ureters limiting endoscopic access, as well as a greater risk of injuring aberrant vessels or overlying abdominal viscera. Management of symptomatic hydronephrosis in pregnancy is often treated with ureteric stent placement (12-15). Advantages of treating an obstructed kidney with a percutaneous nephrostomy rather than an indwelling ureteric stent are the lower cost and ease of changes during pregnancy (16). In addition, percutaneous nephrostomies have a lower incidence of irritative LUTS, reduced analgesic requirements and patients are thought to have a better quality of life as compared to those patients with indwelling stents (17,18). In this particular patient, the location of the ectopic kidney was such that a percutaneous nephrostomy could not be placed safely under ultrasound guidance due to proximity of the iliac artery. In order to minimize the risk of radiation exposure to the fetus, the ALARA principle was applied. The fluoroscopy settings during stent placement were set to low dose with collimation and the patient’s gravid abdomen was shielded using a lead gown. Fluoroscopy time was limited as much as possible and there was only a total accumulated dose area product to the patient of 0.7 Gy/cm2.
Any surgery during pregnancy has increased risks. Pregnancy is a hypercoagulable state associated with an increased risk of deep venous thrombosis and thromboembolism and there is also an increased risk of aspiration during general anesthesia. Regarding potential risks to the fetus during surgery, a review of 5,405 patients found no increased incidence of congenital abnormalities, premature labor or stillbirths during non-obstetrical operations (19). Rapid encrustation of ureteric stents as a result of the hypercalciuria and hyperuricosuria during pregnancy must be considered when deciding when to exchange stents (20). This patient had no prior history of urolithiasis so it was decided to extend the time to stent exchange closer to her due date at 34 weeks gestation in case the endoscopic manipulation induced premature labor.
Resolution of the hydronephrosis following delivery confirmed that the obstruction was secondary to the pregnancy and not a previously undiagnosed chronic obstruction such as pelviureteric junction obstruction which is common in ectopic kidneys (4).
Conclusion
Hydronephrosis of pregnancy occurs in most women but it has been found not to affect those patients with pelvic kidneys. This patient illustrates that it is possible for women with ectopic pelvic kidneys to develop hydronephrosis during pregnancy. While conservative management is successful in controlling symptoms in most patients with hydronephrosis during pregnancy, a patient with a pelvic kidney such as this may require more aggressive drainage of the kidney with a ureteric stent or nephrostomy.
Fig.1a
Fig1.b
Fig.2
Acknowledgments
None
Disclosure Statement
No competing financial interests exist.
References
1. Faundes A, Bricola-Filho M, Pinto et al. Dilatation of the urinary tract during pregnancy: Proposal of a curve of maximal caliceal diameter by gestational age. Am J Obstet Gynecol 1998; 178(5):1082-1086.
2. Roberts JA. Hydronephrosis of pregnancy. Urology 1976; 8(1):1-4.
3. Rasmussen PE, Nielsen FR. Hydronephrosis during pregnancy: a literature survey. Eur J Obstet Gynecol Reprod Biol 1988; 27(3):249-259.
4. Cinman NM, Okeke Z, Smith AD. Pelvic Kidney: Associated Diseases and Treatment. J Endourology 2007; 21(8):836-842.
5. Zafar FS, Lingeman JE. Value of laparoscopy in the management of calculi complicating renal malformations. J Endourol 199; 10(4):379-383.
6. Malhorta N, Roy KK, Garg PK, et al. Ectopic hydronephrotic kidney masquerading as an ovarian cyst during pregnancy. Eur J Obstet Gynecol Reprod Biol 2001; 97(2):239-240.
7. van Wagenen G, Jenkins RH. An experimental examination of factors causing ureteral dilation of pregnancy. J Urol 1939; 42:1010-1020.
8. Lubin S, Drexler LS, Bilotta WA. Post-partum pyeloureteral changes following hormone administration. Surg Gynecol Obstet 1941; 73:391.
9. Swanson SK, Heilman RL, Eversman WG. Urinary tract stones in pregnancy. Surg Clin North Am 1995; 75:123-142.
10. McAleer SJ, Loughlin KR. Nephrolithiasis and pregnancy. Curr Opin Urol 2004; 14:123-127.
11. Harrow BR, Sloane JA, Salhanith L. Etiology of hydronephrosis of pregnancy. Surg Gynecol Obstet 1964; 119:1042-1048.
12. Sadan O, Berar M, Sagiv R, et al. Ureteric stent in severe hydronephrosis of pregnancy. Eur J Obstet Gynecol Reprod Biol 1994; 56(2):79-81.
13. Delakas D, Karyotis I, Loumbakis P, et al. Ureteral drainage by Double-J-catheters during pregnancy. Clin Exp Obstet Gynecol 2000; 27(3-4):200-202.
14. Fainaru O, Almog B, Gamzu R, et al. The Management of Symptomatic Hydronephrosis in pregnancy. BJOG 2002; 109:1385-1387.
15. Vendola N, Giumelli P, Galdini R, et al. Ureteral Drainage with Double-J Catheters in Obstructive Uropathy during Pregnancy. Gynecol Obstet Invest 1995; 40:274-275.
16. Kavoussi LR, Albala DM, Basler JW, et al. Percutaneous Management of Urolithiasis During Pregnancy. J Urol 1992; 148(3):1069-1071.
17. Joshi HB, Adams S, Obadeyi OO, et al. Nephrostomy tube or “JJ” ureteric stent in ureteric obstruction: assessment of patient perspectives using quality of life survey and utility analysis. Eur Urol 2001; 39:695-701.
18. Mokhmalji H, Braun PM, Portillo JM, et al. Percutaneous nephrostomy versus ureteral stents for diversion of hydronephrosis caused by stones: a prospective randomized clinical trial. J Urol 2001; 165:1088-1092.
19. Mazze RI, Kallen B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Amer J Obst Gynec 1989, 161:1178-1185.
20. Goldfarb R, Nerrhut G, Lederer E. Management of acute hydronephrosis of pregnancy by ureteral stenting: risk of stone formation. J Urol 1989; 141:921-922.
Date added to bjui.org: 08/12/2012
DOI: 10.1002/BJUIw-2012-058-web