Article of the Week: Effect of MetS on serum PSA levels is concealed by enlarged prostate
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video discussing the paper.
If you only have time to read one article this week, it should be this one.
Actual lowering effect of metabolic syndrome on serum prostate-specific antigen levels is partly concealed by enlarged prostate: results from a large-scale population-based study
Abstract
Objectives
To clarify the lowering effect of metabolic syndrome (MetS) on serum prostate-specific antigen (PSA) levels in a Chinese screened population.
Subjects and Methods
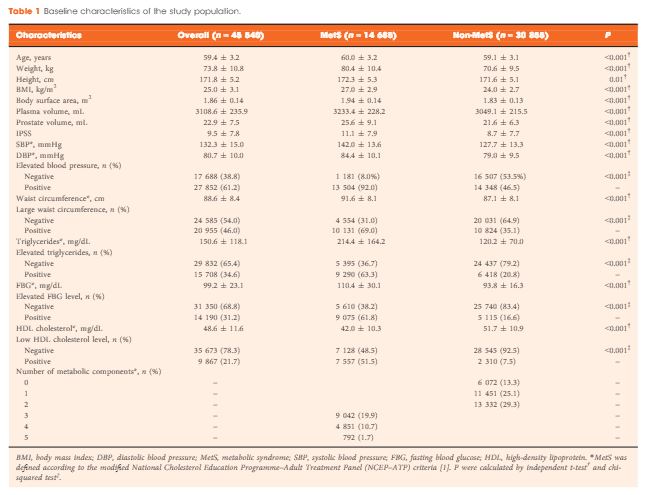
A total of 45 540 ostensibly healthy men aged 55–69 years who underwent routine health check-ups at Beijing Shijitan Hospital between 2008 and 2015 were included in the study. All the men underwent detailed clinical evaluations. PSA mass density was calculated (serum PSA level × plasma volume ÷ prostate volume) for simultaneously adjusting plasma volume and prostate volume. According to the modified National Cholesterol Education Programme–Adult Treatment Panel (NCEP-ATP) III criteria, patients were dichotomized by the presence of MetS, and differences in PSA density and PSA mass density were compared between groups. Linear regression analysis was used to evaluate the effect of MetS on serum PSA levels.
Results
When larger prostate volume in men with MetS was adjusted for, both PSA density and PSA mass density in men with MetS were significantly lower than in men without MetS, and the estimated difference in mean serum PSA level between men with and without MetS was greater than that before adjusting for prostate volume. In the multivariate regression model, the presence of MetS was independently associated with an 11.3% decline in serum PSA levels compared with the absence of MetS. In addition, increasing number of positive MetS components was significantly and linearly associated with decline in serum PSA levels.
Conclusion
The actual lowering effect of MetS on serum PSA levels was partly concealed by the enlarged prostate in men with MetS, and the presence of MetS was independently associated with lower serum PSA levels. Urologists need to be aware of the effect of MetS on serum PSA levels and should discuss this subject with their patients.