Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by prominent members of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma – results from a comprehensive multi-centre database (CORONA/SATURN-Project)
Sabine D. Brookman-May1, Matthias May2, Shahrokh F. Shariat3, Giacomo Novara4, Richard Zigeuner5, Luca Cindolo6, Ottavio De Cobelli7, Cosimo De Nunzio8, Sascha Pahernik9, Manfred P. Wirth10, Nicola Longo11, Alchiede Simonato12, Sergio Serni13, Salvatore Siracusano14, Alessandro Volpe15, Giuseppe Morgia16, Roberto Bertini17, Orietta Dalpiaz5, Christian Stief1, and Vincenzo Ficarra4,18; Members of the CORONA-Project, the SATURN-Project, and the Young Academic Urologists Renal Cancer Group
1Department of Urology, Ludwig-Maximilians-University Munich, Campus Grosshadern, Munich, 2Department of Urology, St. Elisabeth Hospital Straubing, Straubing, Germany, 3Department of Urology and Division of Medical Oncology, Weill Cornell Medical College, New York, NY, USA, 4Department of Urology, University of Padua, Padua, Italy, 5Department of Urology, Medical University of Graz, Graz, Austria, 6Department of Urology, S. Pio Da Pietrelcina Hospital, Vasto, 7Department of Urology, European Institute of Oncology, Milan, 8Department of Urology, S. Andrea Hospital, Rome, Italy, 9Department of Urology, University of Heidelberg, Heidelberg, 10Department of Urology, Carl Gustav Carus Hospital, University of Dresden, Dresden, Germany, 11Department of Urology, University of Naples Federico II, Napoli, 12‘Luciano Giuliani’ Department of Urology, University of Genoa, Genoa, 13Department of Urology, University of Florence, Careggi Hospital, Florence, 14Department of Urology, University of Trieste, Trieste, 15Department of Urology, University of Eastern Piedmont, Maggiore della Carità Hospital, Novara, 16Departments of Urology, University of Catania, Catania, 17Department of Urology, Vita-Salute University San Raffaele, Milan, 18Department of Urology, Vita-Salute University San Raffaele, Milan, Italy, and OLV Robotic Surgery Institute, Aalst, Belgium
S.D.B.-M. and M.M contributed equally to this manuscript
OBJECTIVES
• To assess the prognostic impact of time to recurrence (TTR) on cancer-specific survival (CSS) after recurrence in patients with renal cell carcinoma (RCC) undergoing radical nephrectomy or nephron-sparing surgery.
• To analyse differences in clinical and histopathological criteria between patients with early and late recurrence.
PATIENTS AND METHODS
• Of 13 107 patients with RCC from an international multicentre database, 1712 patients developed recurrence in the follow-up (FU), at a median (interquartile range) of 50.1 (25–106) months.
• In all, 1402 patients had recurrence at ≤5 years (Group A) and 310 patients beyond this time (Group B).
• Differences in clinical and histopathological variables between patients with early and late recurrence were analysed.
• The influence of TTR and further variables on CSS after recurrence was assessed by Cox regression analysis.
RESULTS
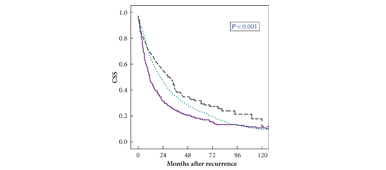
• Male gender, advanced age, tumour diameter and stage, Fuhrman grade 3–4, lymphovascular invasion (LVI), and pN + stage were significantly more frequent in patients with early recurrence, who had a significantly reduced 3-year CSS of 30% compared with patients in Group B (41%; P = 0.001).
• Age, gender, tumour histology, pT stage, and continuous TTR (hazard ratio 0.99, P = 0.006; monthly interval) independently predicted CSS.
• By inclusion of dichotomised TTR in the multivariable model, a significant influence of this variable on CSS was present until 48 months after surgery, but not beyond this time.
CONCLUSIONS
• Advanced age, male gender, larger tumour diameters, LVI, Fuhrman grade 3–4, pN + stage, and advanced tumour stages are associated with early recurrence.
• Up to 4 years from surgery, a shorter TTR independently predicts a reduced CSS after recurrence.