Video: Survival after RP for clinically localised prostate cancer
Survival after radical prostatectomy for clinically localised prostate cancer: a population-based study
Martin Andreas Røder1, Klaus Brasso1, Ib Jarle Christensen2, Jørgen Johansen3, Niels Christian Langkilde4, Helle Hvarness1, Steen Carlsson5, Henrik Jakobsen6, Michael Borre7 and Peter Iversen1
OBJECTIVES
• To describe survival and cause of death in a nationwide cohort of Danish patients with prostate cancer undergoing radical prostatectomy (RP).
• To describe risk factors associated with prostate cancer mortality.
PATIENTS AND METHODS
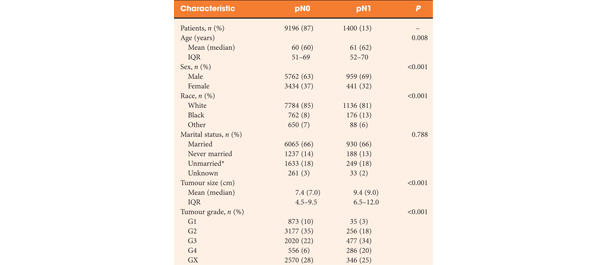
• Observational study of 6489 men with localised prostate cancer treated with RP at six different hospitals in Denmark between 1995 and 2011.
• Survival was described using Kaplan–Meier estimates. Causes of death were obtained from the national registry and cross-checked with patient files.
• Cumulative incidence of death, any cause and prostate cancer-specific, was described using Nelson–Aalen estimates.
• Risk for prostate cancer death was analysed in a Cox multivariate regression model using the covariates: age, cT-category, PSA level and biopsy Gleason score.
RESULTS
• The median follow-up was 4 years. During follow-up, 328 patients died, 109 (33.2%) from prostate cancer and 219 (66.8%) from other causes. Six patients (0.09%) died ≤30 days of RP.
• In multivariate analysis, cT-category was a predictor of prostate cancer death (P < 0.001). Compared with T1 disease, both cT2c (hazard ratio [HR] 2.2) and cT3 (HR 7.2) significantly increased the risk of prostate cancer death. For every doubling of PSA level the risk of prostate cancer death was increased by 34.8% (P < 0.001). Biopsy Gleason score 4 + 3 and ≥8 were associated with an increased risk of prostate cancer death compared with biopsy Gleason score ≤ 6 of 2.3 and 2.7 (P = 0.003), respectively.
• The cumulative hazard of all-cause and prostate cancer-specific mortality after 10 years was 15.4% (95% confidence interval [CI] 13.2–17.7) and 6.6% (95% CI 4.9–8.2) respectively.
CONCLUSIONS
• We present the first survival analysis of a complete, nationwide cohort of men undergoing RP for localised prostate cancer.
• The main limitation of the study was the relatively short follow-up.
• Interestingly, our national results are comparable to high-volume, single institution, single surgeon series.