Cowbells and conundrums – the 3rd Advanced Prostate Cancer Consensus Conference

by Professor Declan G Murphy
Urologist & Director of Genitourinary Oncology, Peter MacCallum Cancer Centre, Melbourne, Australia
Twitter: @declangmurphy

The 3rd Advanced Prostate Cancer Consensus Conference (APCCC) took place in Basel in late August 2019, and the subsequent manuscript was published in European Urology just recently. We delayed posting this blog until now as the recommendations were under embargo until the manuscript went online. As with the previous two APCCC events (which took place in St Gallen; the so-called “St Gallen meetings”), this “Basel meeting” and its resultant recommendations are certain to provoke discussion due to the contentious nature of the topics which feature. Indeed, much of the raison d’etre of the meeting is to create recommendations from key opinion leaders to help guide decision-making in prostate cancer, particularly in areas where confusion exists, and where traditional guidelines are not clear.
The format is as follows:
- The meeting takes place every two years and includes two full days of plenary sessions from world leaders, and one half-day of voting to try and achieve consensus on hot topics
- 72 of the world’s leading experts in prostate cancer are invited to deliver plenary addresses, and more than 750 delegates from 65 different countries
- Ten areas of controversy are featured in the plenary sessions, and invited experts participate in a live vote on the final day to see if consensus can be reached. More than 120 questions are selected by the panel over the previous few months.
- The level of consensus was defined as follows: answer options with 75% agreement were considered consensus, and answer options with 90% agreement were considered strong consensus.
- The results are published in a detailed manuscript (40 pages!) in European Urology
The meeting is convened by Dr Silke Gillessen and Dr Aurelius Omlin who are world-renowned experts in prostate cancer. One of the unique and most enjoyable aspects of the APCCC is the unashamed Swiss-ness which Silke and Aurelius bring to the meeting. The meeting is conducted in a very relaxed manner with excellent interaction between the Faculty which is part of the high value of the meeting. Of course, one would expect a meeting in Switzerland to run efficiently, and Silke and Aurelius wield a goat’s bell for the one minute warning; if you hear the cow bell, then the time is up!

As before, the invited panel is a truly global gathering of world experts in prostate cancer:
A truly global gathering of world leaders in prostate cancer
The ten areas of controversy for #APCCC19 are as listed below, followed by a summary of some of the notable areas of consensus, along with some areas of non-consensus.
- Locally advanced prostate cancer
- Biochemical recurrence of prostate cancer after local therapy
- Management of the primary tumour in the metastatic setting
- Management of newly diagnosed metastatic hormone-sensitive prostate cancer (mHSPC), including oligometastatic prostate cancer
- Management of nonmetastatic (M0) castration-resistant prostate cancer (CRPC)
- Management of metastatic CRPC (mCRPC)
- Bone health and bone metastases
- Molecular characterisation of tissue and blood
- Interpatient heterogeneity
- Side effects of hormonal treatments and their management
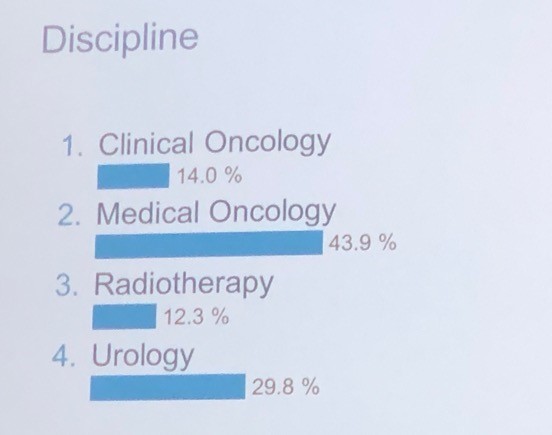
It was interesting to note the proportion of voting panellists by discipline as listed below, in particular the healthy proportion of urologists in a meeting focussed on advanced prostate cancer:
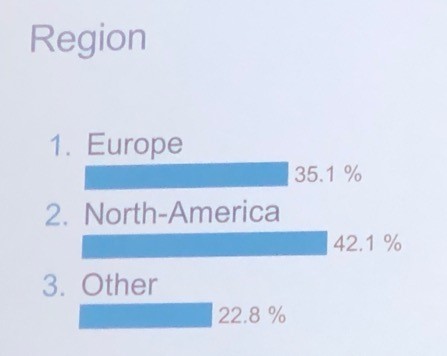
And also by region as listed below.
Obviously a massive amount of territory gets covered during this meeting, but I have highlighted some of the key recommendations within each of the ten areas of controversy below:
Locally advanced prostate cancer:
This section featured plenary addresses on node-positive prostate cancer from myself and radiation oncologist Dr Mack Roach. There was strong consensus that some sort of loco-regional treatment with surgery or radiation (RT) should be offered to men with node-positive prostate cancer, combined with androgen deprivation therapy (ADT) for men undergoing RT. The impact of PSMA PET/CT in defining N1 disease was considered and was recommended for accurate staging (pending the read out of the proPSMA trial which had not yet been published). Regarding duration of ADT, there was no consensus with answers ranging from six months to three years.
Biochemical recurrence (BCR) of prostate cancer after local therapy:
One of the stand-out themes of this year’s APCCC was the impact of PSMA PET/CT for imaging prostate cancer, and Lu-PSMA theranostics as a treatment for mCRPC. I am pleased to say that much of the focus of this was on data from here in Australia, and there was much favourable comment on how Australia has led in this area. It is fair to say there was a reasonable amount of envy also for how much access we have to PSMA PET/CT compared to many other parts of the world. In one of the opening plenaries, Professor Ian Davis did a terrific job overviewing this, and did introduce some cautionary tones about the management impact of novel imaging.
There was consensus that PSMA PET/CT should be used for the assessment of BCR following radiation or surgery. This is a new recommendation compared with the previous meeting, and is in line with the most recent EAU Prostate Cancer Guidelines. What PSA level should we image at? Most discussion centred around a PSA of 0.2ng/mL or greater following surgery.
For patients undergoing salvage RT, there was consensus that this should be accompanied by a short period (4-12 months) of ADT. 83% of panellists voted in favour of offering salvage RT before PSA reaches 0.5ng/mL, with 37% offering RT before PSA reaches 0.2ng/mL. There was consensus that ADT alone should not be used for the majority of patients with rising PSA and no evidence of metastases following prior local therapy.
Management of the primary tumour in the metastatic setting
This was certainly a hot topic. There was much discussion around the role of RT to the primary in men presenting with metastatic prostate cancer (in addition to lifelong ADT), and the most recent data from STAMPEDE led to a strong (98%) consensus for the use of RT in men presenting with low-volume metastatic prostate cancer. Volume was defined based on conventional imaging using classic CHAARTED criteria. The recommended dose was 55Gy over four weeks as per STAMPEDE.
Should we extrapolate from this data and offer surgery as local therapy in the same group of patients? There was 88% consensus that we SHOULD NOT offer surgery, other than within a clinical trial. There was also consensus that patients with N1 disease should be offered RT to the nodes in addition to the primary.
Management of newly diagnosed metastatic hormone-sensitive prostate cancer (mHSPC), including oligometastatic prostate cancer
This is clearly one of the fastest moving areas in advanced prostate cancer and there was much new data to consider. The panellists reached consensus on 12 areas of mHSPC including:
- Nomenclature – there was 77% consensus that we should avoid the term “castration”, although there was not consensus on what other term we should use! In describing treatment-naïve men, it is easy as we can use the term mHSPC. However, when treatment resistance emerges, we are still left with mCRPC (87% consensus).
- I must say there was an outstanding intervention from patient advocate, Mr Millman, after the initial round of voting on this topic, saying how much patients detest the use of the term “castration”. I could not have agreed more. This led to the convenors calling for a repeat vote with subsequent vote in favour of avoiding the use of the term.
- 95% consensus for obtaining histological evidence of prostate cancer in men suspected of having M1 disease; 96% consensus that ADT could be initiated prior to biopsy.
- Most striking – 100% of panellists voted for ADT combined with something else (docetaxel or an androgen receptor (AR) pathway inhibitor) in patients with de novo high-volume mHSPC. There was much discussion about which combination should be offered, but there was no consensus. The decision can be individualised based on patient factors and local registration and reimbursement status. In Australia that means docetaxel for most patients while we awai tregistration/reimbursement for agents such as abiraterone, enzalutamide and apalutamide.
- There was also consensus for combination approaches in men with de novo low-volume mHSPC, with 85% voting in favour of some additional systemic therapy in addition to ADT, and 80% supporting RT to the primary.
- Similarly, in men with relapsing high-volume or low-volume mHSPC, there was consensus to offer combination systemic approaches, with no consensus on which therapy to offer in addition to ADT.
- There was consensus (78%) that in men with mHSPC diagnosed with conventional imaging, that no additional imaging (ie PSMA PET/CT) should be utilised. That horse has already bolted in Australia where it is not unusual for men to be diagnosed with M1 disease using PSMA PET/CT in the first instance.
Regarding oligometastatic disease, there was consensus that if metastasis-directed therapy (MDT) is to be considered, that the extent and location of disease should not be defined using conventional imaging (79%), but should be defined using more sensitive imaging such as PSMA PET/CT (75%). There was also strong consensus that a distinction should be made between lymph node-only disease, and M1 disease involving other sites. Systemic therapy should be used in addition to local therapy to all local sites of disease (75%). I must say that I was pretty surprised that consensus was reached on this point, as guidelines still suggest that MDT approaches should still only be offered within clinical trials.
Management of nonmetastatic (M0) castration-resistant prostate cancer (CRPC)
What even is M0 CRPC? Once again, PSMA PET/CT dominated the conversation. Although there is a relatively recently accepted definition of high-risk M0 CRPC (castrate levels of testosterone; PSA doubling time of </= 10 months; M0 based on conventional imaging), it is fair to say that there was much interest in the role of PSMA PET/CT in this population of patients. Data about to be published at the time of the meeting reported that 98% of patients in this setting will have identifiable disease on PSMA PET/CT despite being M0/N0 on conventional imaging. There these patients are actually mCRPC, rather than M0 CRPC, albeit based on novel imaging with a lead-time bias. Nonetheless, following various overviews of the recent pivotal data showing improvements in metastasis-free survival (MFS, conventional imaging) in patients with M0 CRPC receiving enzalutamide, apalutamide or darolutamide, the panel voted 86% in favour of using one of these agents in this population of high-risk M0 CRPC. We also voted 86% in favour of NOT extrapolating this data to M0 CROC patients with PSA doubling time of greater than 10 months.
Management of metastatic CRPC (mCRPC)
Another huge area with much data to consider. Although much of this had been considered at the previous APCCC and indeed, there were many areas where consensus was not reached eg which agent to use for first-line mCRPC (docetaxel vs AR pathway inhibitor). Despite general enthusiasm for molecular profiling/precision medicine approaches (and some outstanding talks on these areas), there was 85% consensus that we should not use AR-V7 status when considering mCRPC patients for abiraterone or enzalutamide. There was consensus that a steroid dose of prednisone 5mg bd should be used when starting mCRPC patients on abiraterone, and an 86% consensus that a tapering course of steroids should be used when discontinuing abiraterone or docetaxel.
There was considerable interest in the role of reduced dose abiraterone (250mg with food, instead of 1000mg without food), based on a phase II study, and the panel voted 86% in favour of a reduced dose regimen when there are resource or patient constraints on receiving the full dose.
One of the standout talks of the meeting was delivered by Prof Michael Hofman on the role of Lu-PSMA in progressive mCRPC as he presented data from the phase II trial at Peter Mac published in Lancet Oncology (to date, the only prospective data on Lu-PSMA), and on the TheraP randomised controlled trial from Australia which will read out at ASCO in June this year. For patients with PSMA imaging-positive mCRPC who have exhausted approved treatments and cannot enrol in clinical trials, 43% of panellists voted for Lu-PSMA therapy in the majority of patients, and 46% voted for it in a minority of selected patients. For selecting patients for 177Lu-PSMA therapy, 64% of panellists voted for PSMA PET/CT plus FDG PET/CT with or without standard imaging, 21% voted for PSMA PET/CT plus standard imaging, and 15% voted for PSMA PET/CT alone. Although consensus was not reached on this issue, it was clear that the panel were very influenced by Michael’s excellent presentation on this topic, highlighting observations in the Peter Mac phase II trial that the use of FDG PET/CT in addition to PSMA PET/CT led to enhanced patient selection for Lu-PSMA therapy.
Bone health and bone metastases
There was 77% consensus in favour of routine screening for osteoporosis risk factors (e.g. current/history of smoking, corticosteroids, family history of hip fracture, personal history of fractures, rheumatoid arthritis, 3 alcohol units/day, and BMI), in patients with prostate cancer starting on long-term ADT. There was 86% consensus that mCRPC patients with predominantly bone disease and without visceral metastases, should be considered for radium-223 therapy, although this hardly applies to Australia where radium-223 is difficult to access and not reimbursed (plus Lu-PSMA available).
Molecular characterisation of tissue and blood
The plenaries on this topic were some of the most stimulating of the whole meeting. Truly outstanding talks from the pre-eminent leaders in the world. Among the consensus areas were:
- 90% support for the assessment of germline BRCA1/2 status in M1 prostate cancer patients at some stage of the disease
- 94% consensus that mismatch repair status (MSI high) should be assessed at some stage in M1 disease, most likely in mCRPC.
- 96% consensus that PD-1 inhibition should be considered for MSI high patients at some stage in the disease course
- Strong consensus (93%) for PARP inhibitor or platinum therapy at some point during the disease course in patients with a deleterious germline BRCA1/2 mutation
- Genetic counselling and/or germline DNA testing for patients with newly diagnosed metastatic (M1) castration-sensitive/naïve prostate cancer: consensus (84%) for genetic counselling and/or germline DNA testing for the majority of patients with newly diagnosed metastatic prostate cancer.
Interpatient heterogeneity
There were some terrific talks on heterogeneity in prostate cancer, including ethnic and regional diversity, and the assessment and management of older patients. This year’s meeting expanded on this section compared to previously to acknowledge the diversity of prostate cancer around the world, and the fact that much of the data used to make recommendations is based on particular patient cohorts. The panel did reach consensus (76%) for the extrapolation of efficacy data to patients older than the majority of patients enrolled in a trial.
Side effects of hormonal treatments and their management
Professor Mark Frydenberg was one of the invited plenary speakers in this session and did a terrific job overviewing the management of hot flushes. I have not seen this topic discussed better anywhere in the world. There were also terrific talks on strategies to mitigate other side-effects. The panel reached strong consensus (94%) for the use of resistance and aerobic exercise to reduce fatigue in patients receiving systemic therapy for prostate cancer (apart from therapy dose reduction if possible).


Need more detail?
If you are interested in more detail, please download the manuscript from European Urology (open access), or visit Urotoday where the plenary lectures are available, along with exclusive interviews with many of the invited experts.
Finally, the 4th APCCC will take place from 7-9th October 2021. It will take place in the beautiful city of Lugano towards the Italian side of Switzerland. I encourage anyone with a strong interest in prostate cancer to consider attending. It will be a most stimulating and enjoyable few days immersed in the world of prostate cancer, and conducted with wonderful Swiss hospitality once again by the fabulous Silke Gillessen and Aurelius Omlin.







