Article of the week: Discovery provides new means for regenerative bladder reconstruction
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video of Dr Verdi discussing his paper.
If you only have time to read one article this week, it should be this one.
Endometrial stem cell differentiation into smooth muscle cell: a novel approach for bladder tissue engineering in women
Alireza Shoae-Hassani*, Shiva Sharif, Alexander M. Seifalian†, Seyed Abdolreza Mortazavi-Tabatabaei‡, Sassan Rezaie§ and Javad Verdi
Departments of Applied Cell Sciences and §Medical Biotechnology, School of Advanced Technologies in Medicine, and *Department of Stem cell and Tissue Engineering, Research Center for Science and Technology in Medicine (RCSTiM), Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran, †University College London, UCL Centre for Nanotechnology and Regenerative Medicine, London, UK, and ‡Proteomics Research Center, Faculty of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic of Iran
OBJECTIVE
• To investigate manufacturing smooth muscle cells (SMCs) for regenerative bladder reconstruction from differentiation of endometrial stem cells (EnSCs), as the recent discovery of EnSCs from the lining of women’s uteri, opens up the possibility of using these cells for tissue engineering applications, such as building up natural tissue to repair prolapsed pelvic floors as well as building urinary bladder wall.
MATERIALS AND METHODS
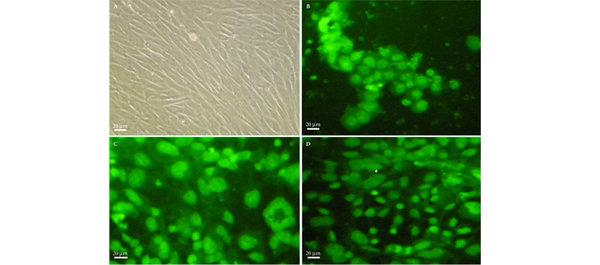
• Human EnSCs that were positive for cluster of differentiation 146 (CD146), CD105 and CD90 were isolated and cultured in Dulbecco’s modified Eagle/F12 medium supplemented with myogenic growth factors.
• The myogenic factors included: transforming growth factor β, platelet-derived growth factor, hepatocyte growth factor and vascular endothelial growth factor.
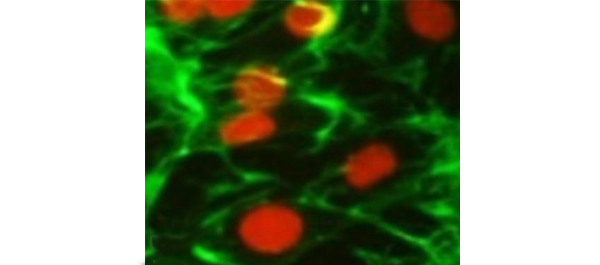
• Differentiated SMCs on bioabsorbable polyethylene-glycol and collagen hydrogels were checked for SMC markers by real-time reverse-transcriptase polymerase chain reaction (RT-PCR), western blot (WB) and immunocytochemistry (ICC) analyses.
RESULTS
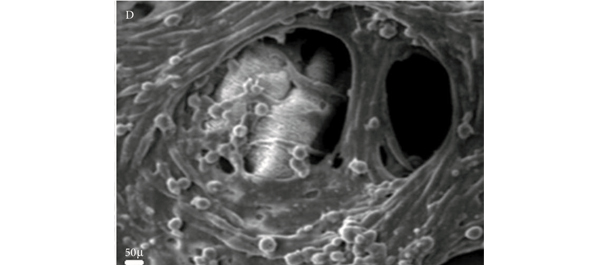
• Histology confirmed the growth of SMCs in the hydrogel matrices.
• The myogenic growth factors decreased the proliferation rate of EnSCs, but they differentiated the human EnSCs into SMCs more efficiently on hydrogel matrices and expressed specific SMC markers including α-smooth muscle actin, desmin, vinculin and calponin in RT-PCR, WB and ICC experiments.
• The survival rate of cultures on the hydrogel-coated matrices was significantly higher than uncoated cultures.
CONCLUSIONS
• Human EnSCs were successfully differentiated into SMCs, using hydrogels as scaffold.
• EnSCs may be used for autologous bladder wall regeneration without any immunological complications in women.
• Currently work is in progress using bioabsorbable nanocomposite materials as EnSC scaffolds for developing urinary bladder wall tissue.
Read Previous Articles of the Week