Article of the Week: Focal irreversible electroporation as primary treatment for localized prostate cancer
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Focal irreversible electroporation as primary treatment for localized prostate cancer
Abstract
Objectives
To determine the safety, quality of life (QoL) and short‐term oncological outcomes of primary focal irreversible electroporation (IRE) for the treatment of localized prostate cancer (PCa), and to identify potential risk factors for oncological failure.
Patients and Methods
Patients who met the consensus guidelines on patient criteria and selection methods for primary focal therapy were eligible for analysis. Focal IRE was performed for organ‐confined clinically significant PCa, defined as high‐volume disease with Gleason sum score 6 (International Society of Urological Pathology [ISUP] grade 1) or any Gleason sum score of 7 (ISUP grades 2–3). Oncological, adverse event (AE) and QoL outcome data, with a minimum of 6 months’ follow‐up, were analysed. Patient characteristics and peri‐operative treatment variables were compared between patients with and without oncological failure on follow‐up biopsy. Wilcoxon’s signed rank test, Wilcoxon’s rank sum test and the chi‐squared test were used to assess statistically significant differences in paired continuous, unpaired continuous and categorical variables respectively.
Results
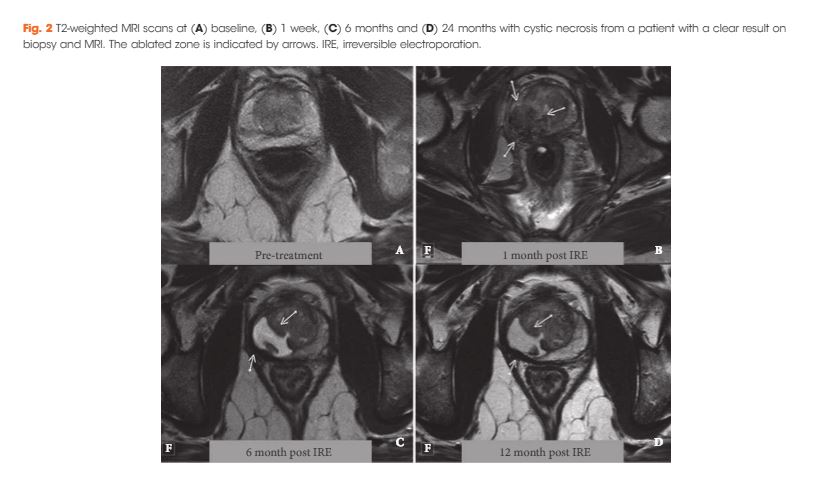
A total of 63 patients met all eligibility criteria and were included in the final analysis. No high‐grade AEs occurred. QoL questionnaire analysis demonstrated no significant change from baseline in physical (P = 0.81), mental (P = 0.48), bowel (P = 0.25) or urinary QoL domains (P = 0.41 and P = 0.25), but there was a mild decrease in the sexual QoL domain (median score 66 at baseline vs 54 at 6 months; P < 0.001). Compared with baseline, a decline of 70% in prostate‐specific antigen level (1.8 ng/mL, interquartile range 0.96–4.8 ng/mL) was seen at 6–12 months. A narrow safety margin (P = 0.047) and system errors (P = 0.010) were identified as potential early risk factors for in‐field oncological failure. In‐field and whole‐gland oncological control on follow‐up biopsies was 84% (38/45 patients) and 76% (34/45 patients); this increased to 97% (38/39 patients) and 87% (34/39 patients) when patients treated with a narrow safety margin and system errors were excluded.
Conclusion
Our data support the safety and feasibility of focal IRE as a primary treatment for localized PCa with effective short‐term oncological control in carefully selected men.