Editorial: The ATOMS… Is it really all so easy?
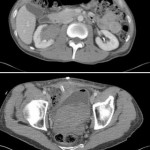
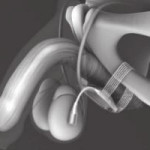
The adjustable transobturator male system (ATOMS® Agency for Medical Innovations A.M.I., Feldkirch, Austria) is an interesting device (the design is evocative of the historical Kaufmann sling) that is now incorporated into the wide spectrum of post-prostatectomy incontinence treatments, and which confirms the real existing pathology despite the introduction of optimised robot-assisted prostate surgery. An interesting article in the present issue of BJUI describes the long-term outcome of the ATOMS device [1].
But there are some considerations that I would like to raise about this device. Only the first generation of ATOMS (inguinal port, IP) has long-term follow-up data and 15% of implanted devices have been explanted within 6–33 months [2]. So, I think we have to be prudent and wait for further long-term results for the third-generation silicone-covered scrotal port (SSP) system, as it has only 2–7 months of previously published outcome data.
As the explantation rates of all other silicone devices are still high, between 12% and 35% in the mid- to long-term, why should we not expect this for the ATOMS SSP third generation? All devices (sphincters, Argus, Reemex etc.) have an explantation risk and the causes are, in most cases infections and erosions, i.e. still trophic problems.
As stated, the explant rate of silicone devices is still high, but the removal of silicone parts is relatively easy because of the ‘coating’ by a collagenous capsule. On the other hand, male slings (mesh) are really rarely infected and require explantation, but if this happens a complete removal of the transobturator tape (TOT) male mesh sling is needed, which is still relatively challenging surgery and incapacitating for the patient, especially if the mesh has become ingrown.
Another point in the study of Friedl et al. [1] is that titanium intolerance is described as one of the main causes of device explantation (41%). We have to consider the broad use of titanium-covered meshes or titanium artificial hips, as in the literature prosthesis explantation caused by titanium intolerance have not been reported at such high percentages. It thus seems unlikely that titanium intolerance should be the problem rather than trophic or mechanical factors leading to erosion (41% of ATOMS explants in long-term follow-up [1]).
Lastly, we know that patients with minimal and moderate incontinence can be treated successfully by a simple tissue-fixed TOT sling. Patients with more intrinsic sphincter deficiency, with repeated anastomotic incisions or resections need a more compressive device, and compression needs adjustability over time, a field for devices such as Argus and Reemex for example. Also at night time, patients with incontinence (real and severe intrinsic sphincter deficiency) need an artificial sphincter for continence and good unobstructed voiding (on/off). So which group of patients should be targeted for this combination between a hydraulic-filled balloon, adjustable subcutaneous port and TOT sling, as 60–70% of patients with mild-to-moderate incontinence are dry using the sling alone [3]. Should it be the first choice for all non-irradiated patients? or for those that have TOT failure? Or artificial sphincter failure? Or intrinsic sphincter deficiency? Therefore, what we need to clarify is the real indications for this combined tri-component device (silicone port, silicone balloon and TOT mesh). And we must also not forget that there could be atrophy of the membranous urethra due to the bulking effect of the silicone balloon. This is another point that has to be considered.
In the treatment of male incontinence we must focus not only on the effectiveness but also on the safety of the device used. This is particularly important considering the warning issued by the USA Food and Drug Administration (FDA) for female mesh slings.
How to Cite
Gozzi, C. (2017), Considerations about the adjustable transobturator male system (ATOMS®) device… is it really all so easy?. BJU International, 119: 655–656. doi: 10.1111/bju.13830