Editorial: The Jury on Posterior Muscolofascial Reconstruction is still out
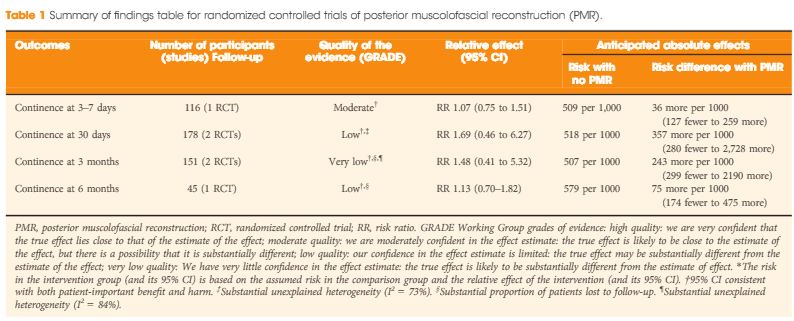
In their systematic review and meta-analysis, Grasso et al. [1] address the question of whether posterior muscolofascial reconstruction (PMR), the so-called Rocco stitch, positively affects urinary continence after radical prostatectomy. The relevance of the question to this structured form of inquiry is that individual studies to date have been inconclusive. We recognize Sir Archie Cochrane, who gave his name to the Cochrane Collaboration that pioneered the methods for conducting systematic reviews, for emphasizing the critical importance of looking at the entire body of evidence in a structured manner when seeking to answer a clinical question [2]. In the present study, which included both randomized controlled trials (RCTs) and observational studies of variable methodological quality, a favourable impact of PMR across all postoperative time points (3–7 days, 30 days, 3 and 6 months) was observed. The effect was most pronounced early on at the time of catheter removal, when the patients undergoing PMR were nearly twice as likely as the control group (risk ratio 1.9; 95% CI 1.3–2.9) to be continent, thereby suggesting a major benefit of this approach. It should be noted, however, that this analysis was dominated by the observational studies, particularly retrospective observational studies, which offer the least degree of methodological rigor.
Even more important, therefore, than the act of pooling across studies is the rating of the quality of evidence for the body of evidence on an outcome-specific basis. Based on the GRADE approach, which has become the most widely endorsed framework for rating the quality of evidence, we would initially place a high and low level of confidence in a body of evidence drawn from RCTs and observational studies, respectively [3]. As a result, one might plan a separate analysis of those two groups of studies first, and only move to pool them if their results were similar. In this case, the results from the RCTs and observational studies were different, with prospective and retrospective studies reporting larger, probably exaggerated effect sizes; however, it is also understood that other aspects such as study limitation (risk of bias), inconsistency, impression, indirectness and risk publication bias may lower our confidence in the effect estimates from RCTs [4]. Focusing on the body of evidence from RCTs alone (Table 1) we have ‘moderate’ confidence that PMR may not improve early continence at the time of catheter removal. Similarly, the few RCTs that contributed to the assessment of continence at later timepoints do not provide evidence that continence is affected favourably, although our confidence for those outcomes is only ‘low’ or ‘very low’, suggesting that future trials may change these estimates of effect. Meanwhile, it should be noted that none of the RCTs appeared to provide information on the potential downsides of PMR, such as rates of urinary retention or bladder neck contracture. As a result, enough uncertainty remains to state that the jury on PMR is still out; this is consistent with the authors’ call for a future high-quality trial, which is reportedly ongoing. While PMR is already widely used by open and robot-assisted prostatectomy surgeons around the globe, this example sheds light on current evidentiary standards of surgical innovation. Following the IDEAL recommendations, it would be much preferred if the urological community committed to well designed trials for novel surgical approaches and device-dependent interventions up front, before moving to widespread dissemination [5].