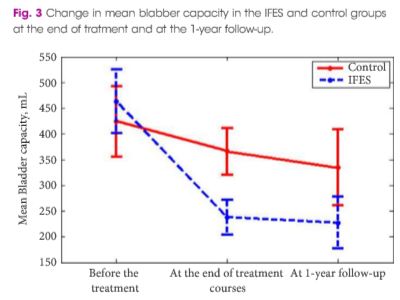
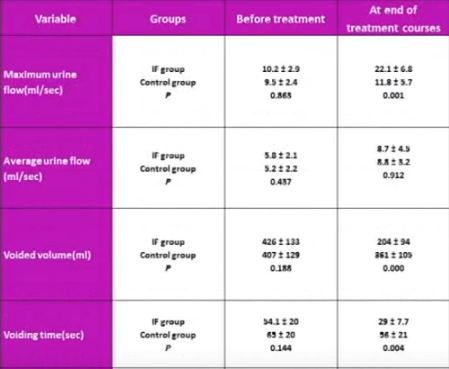
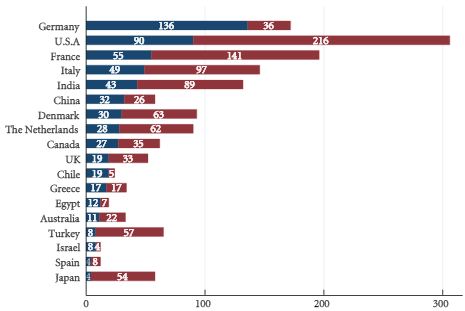
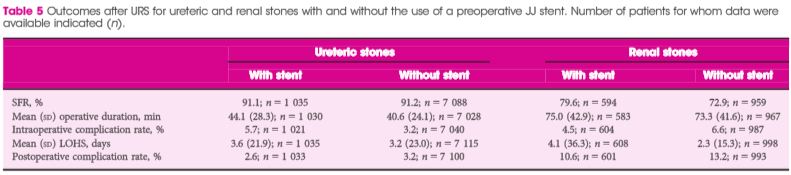
Article of the Week: 68Ga-PSMA has high detection rate of PCa recurrence after RP
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
68Ga-PSMA has high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment
Objectives
To examine the detection rates of 68Ga-PSMA-positron emission tomography (PET)/computed tomography (CT) in patients with biochemical recurrence (BCR) after radical prostatectomy (RP), and also the impact on their management.
Materials and Methods
A total of 300 consecutive patients with prostate cancer (PCa) who underwent 68Ga-PSMA-PET/CT between February and July 2015 were prospectively included in the Prostate Cancer Imaging (ProCan-I) database. For the present analysis, we included patients with BCR (prostate-specific antigen [PSA] level ≥0.05 and <1.0 ng/mL) after RP, who were being considered for salvage radiation therapy (RT) according to the Faculty of Radiation Oncology Genito-Urinary Group (FROGG) guidelines. Two readers assessed each 68Ga-PSMA-PET/CT, and all positive lesions were assigned to an anatomical location. For each patient, the clinical and pathological features were recorded, their association with pathological 68Ga-PSMA uptake was investigated, and detection rates were determined according to PSA level.
Results
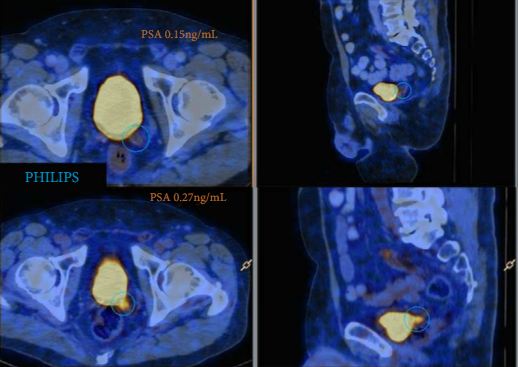
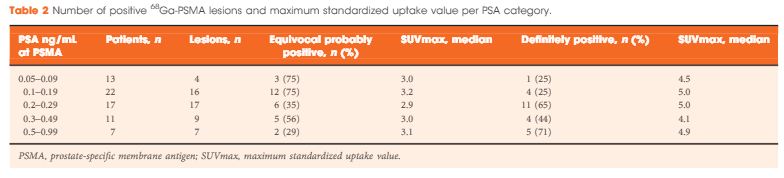
A total of 70 patients were included, and 53 positive 68Ga-PSMA lesions were detected in 38 (54%) patients. Among patients with PSA levels 0.05–0.09 ng/mL, 8% were definitely positive; the corresponding percentages for the other PSA ranges were as follows: PSA 0.1–0.19 ng/mL, 23%; PSA 0.2–0.29 ng/mL, 58%; PSA 0.3–0.49 ng/mL, 36%; and PSA 0.5–0.99 ng/mL, 57%. Eighteen of 70 patients (27%) had pathological 68Ga-PSMA uptake in the prostatic fossa, 11 (14.3%) in the pelvic nodes, and five (4.3%) in both the fossa and pelvic lymph nodes. Finally, there was uptake outside the pelvis with or without a lesion in the fossa or pelvic lymph nodes in four cases (8.6%). As a result of the 68Ga-PSMA findings there was a major management change in 20 (28.6%) patients.
Conclusions
68Ga-PSMA appears to be useful for re-staging of PCa in patients with rising PSA levels who are being considered for salvage RT even at PSA levels <0.5 ng/mL. These results underline the need for further prospective trials to evaluate the changes in RT volume or management attributable to 68Ga-PSMA findings.