Posts
Article of the week: Salvage radical prostatectomy following focal therapy: functional and oncological outcomes
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to this post, there is an editorial written by prominent members of the urological community. Please use the comment buttons below to join the conversation.
If you only have time to read one article this week, we recommend this one.
Salvage radical prostatectomy following focal therapy: functional and oncological outcomes
Jaime O. Herrera-Caceres*, Gregory J. Nason*, Noelia Salgado-Sanmamed†, Hanan Goldberg*, Dixon T.S. Woon*, Thenappen Chandrasekar*, Khaled Ajib*, Guan Hee Tan*, Omar Alhunaidi*, Theodorus van der Kwast‡, Antonio Finelli*, Alexandre R. Zlotta*, Robert J. Hamilton*, Alejandro Berlin†, Nathan Perlis* and Neil E. Fleshner*
*Division of Urology, Department of Surgical Oncology, †Department of Radiation Oncology, and ‡Department of Pathology and Laboratory Medicine, University Health Network, University of Toronto, Toronto, ON, Canada
Abstract
Objectives
To report the oncological and functional outcomes of salvage radical prostatectomy (sRP) after focal therapy (FT).
Patients and Methods
A retrospective review of all patients who underwent sRP after FT was performed. Clinical and pathological outcomes focussed on surgical complications, oncological, and functional outcomes.
Results
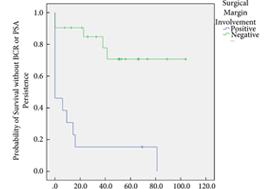
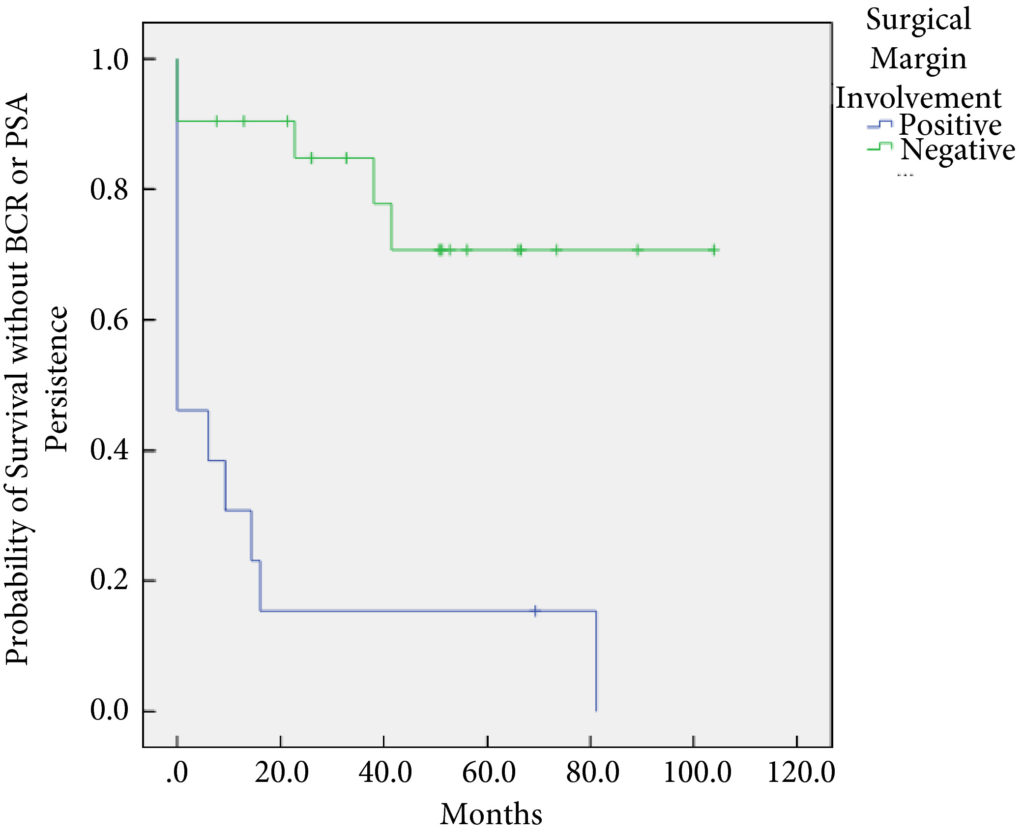
In all, 34 patients were identified. The median (interquartile range [IQR]) age was 61 (8.25) years. FT modalities included high‐intensity focussed ultrasound (19 patients), laser ablation (13), focal brachytherapy (one) and cryotherapy (one). The median (IQR) time from FT to recurrence was 10.9 (17.6) months. There were no rectal or ureteric injuries. Two (5.9%) patients had iatrogenic cystotomies and four (11.8%) developed bladder neck contractures. The mean (sd) hospital stay was 2.5 (2.1) days. The T‐stage was pT2 in 14 (41.2%) patients, pT3a in 16 (47.1%), and pT3b in four (11.8%). In all, 13 (38%) patients had positive surgical margins (PSMs). Six (17.6%) patients received adjuvant radiotherapy (RT). At a mean follow‐up of 4.3 years, seven (20.6%) patients developed biochemical recurrence (BCR), and of these, six (17.6%) patients required salvage RT. PSMs were associated with worse BCR‐free survival (hazard ratio 6.624, 95% confidence interval 2.243–19.563; P < 0.001). The median (IQR) preoperative International Prostate Symptom Score and International Index of Erectile Function score was 7 (4.5–9.5) and 23.5 (15.75–25) respectively, while in the final follow‐up the median (IQR) values were 7 (3.5–11) and 6 (5–12.25), respectively (P = 0.088 and P < 0.001). At last follow‐up, 31 (91.2%) patients were continent, two (5.9%) had moderate (>1 pad/day) incontinence, and one (2.9%) required an artificial urinary sphincter.
Conclusions
sRP should be considered as an option for patients who have persistent clinically significant prostate cancer or recurrence after FT. PSMs should be recognised as a risk for recurrent disease after sRP.
Article of the Week: Impact of 68Ga-PSMA PET/CT in PCa with rising PSA
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
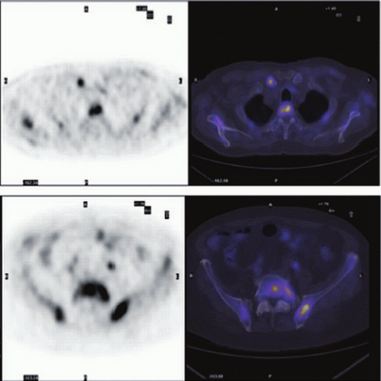
Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach
How to Cite
Albisinni, S., Artigas, C., Aoun, F., Biaou, I., Grosman, J., Gil, T., Hawaux, E., Limani, K., Otte, F.-X., Peltier, A., Sideris, S., Sirtaine, N., Flamen, P. and van Velthoven, R. (2017), Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU International, 120: 197–203. doi: 10.1111/bju.13739
Abstract
Objective
To assess the impact of a novel molecular imaging technique, 68Ga-(HBED-CC)-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT), in the clinical management of patients with prostate cancer with rising prostate-specific antigen (PSA) after treatment with curative intent.
Patients and Methods
In all, 131 consecutive patients were referred to our centre for a 68Ga-PSMA PET/CT in the setting of recurring prostate cancer. Of these patients, 11/131(8%) presented with persistent PSA after radical prostatectomy, while 120/131 (92%) were referred for biochemical recurrence after surgery, radiotherapy or both. The images where taken 1 h after injection of 2 MBq/kg of the 68Ga-(HBED-CC)-PSMA ligand. All examinations were interpreted by two experienced nuclear medicine specialists. Using the results of the examination, a multidisciplinary oncology committee (MOC) reported on the treatment strategy. A positive impact on clinical management was considered if the examination determined a modification in the treatment strategy compared to the MOC decision before PSMA imaging.
Results
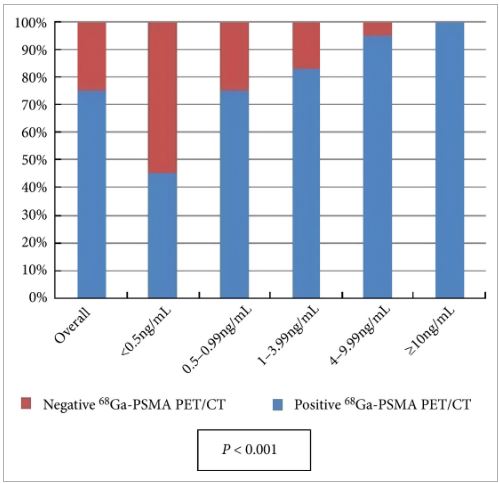
All patients completed the examination with no adverse reactions. The median (interquartile range) PSA level at the time of the examination was 2.2 (0.72–6.7) ng/mL. Overall, 68Ga-PSMA PET/CT detected at least one lesion suspicious for prostate cancer in 98/131 (75%) patients. There was an impact on subsequent management in 99/131 patients (76%). The main modifications included continuing surveillance (withholding hormonal therapy), hormonal manipulations, stereotaxic radiotherapy, salvage radiotherapy, salvage node dissection or salvage local treatment (prostatectomy, high-intensity focussed ultrasound).
Conclusion
Our preliminary experience suggests that performing 68Ga-PSMA PET/CT in patients with prostate cancer with rising PSA after treatment with curative intent can be clinically useful as it changes the treatment strategy in a significant proportion of patients. However, larger prospective trials are needed to validate our present findings.
Editorial: Defining the clinical utility of PSMA-targeted PET imaging of prostate cancer
In the field of oncology, positron emission tomography (PET) is most commonly performed using 2-deoxy-2-[18F]fluoro-d-glucose (18F-FDG), a radiofluorinated glucose analogue that accumulates in cells undergoing aerobic glycolysis. Unfortunately, because of the low glycolytic activity of hormone-naïve prostate cancer cells, 18F-FDG PET has been of little value in imaging men with this malignancy [1]. Instead, clinicians have been left to rely mostly on 99mTc-methylene diphosphonate bone scan, CT, and MRI to stage and follow patients. Recently, however, the development of multiple urea-based small molecules targeting the type II transmembrane glycoprotein prostate-specific membrane antigen (PSMA) has allowed for the highly sensitive and specific detection of prostate cancer using PET imaging [2]. To date, the majority of clinical data with PSMA-targeted PET have been generated with the 68Ga-PSMA-11 radiotracer (also known as 68Ga-PSMA-HBED-CC). Notably, studies evaluating PSMA-targeted PET have mostly focused on establishing the diagnostic performance characteristics of the various radiotracers (e.g. sensitivity and specificity), with relatively few reports exploring the clinical impact or utility of this form of molecular imaging.
In this month’s edition of BJUI, Albisinni et al. [3] aimed to look beyond the performance characteristics of 68Ga-PSMA-11 PET/CT and retrospectively analysed the impact of this imaging test on the management of 131 men with a persistently elevated PSA level or biochemical recurrence after local treatment of their prostate cancer with curative intent. Of these patients, 106 (81%) had undergone a previous radical prostatectomy. The authors defined clinical utility as any imaging finding (or lack thereof) leading to a change in a patient’s pre-PET treatment plan. In total, 68Ga-PSMA-11 PET/CT demonstrated clinical utility in 76% of imaged patients. Most commonly, the results of this imaging test led to avoidance of androgen deprivation therapy (44% of all patients imaged) in place of an alternative management strategy, such as surveillance or salvage radiation therapy. Another notable finding was that among men who had planned to undergo salvage radiation therapy prior to 68Ga-PSMA-11 PET/CT, the majority (19 of 32 [59%]) were managed with an alternative approach after undergoing imaging.
Albisinni et al. [3] are not alone in their observations regarding the high clinical utility of 68Ga-PSMA-11 PET. For example, van Leeuwen et al. [4] previously reported that nearly 30% of men who were felt to be candidates for post-prostatectomy salvage radiation therapy had findings on 68Ga-PSMA-11 PET/CT that led to a major change in management. Additionally, Sterzing et al. [5] found that approximately 50% of patients undergoing radiation therapy planning for primary or recurrent prostate cancer experienced a change to their treatment concept after imaging with 68Ga-PSMA-11 PET/CT. Combined, these data suggest that a substantial proportion of men with prostate cancer stand to have their management altered by undergoing PSMA-targeted PET imaging.
While encouraging, the study by Albisinni et al. is somewhat limited by its retrospective design [3]. An outstanding example of how data on the clinical utility of an imaging test can be prospectively collected comes to us from the National Oncology PET Registry (NOPR) in the USA. Working in collaboration with the Centers for Medicare and Medicaid Services (CMS), NOPR was established to assess the question of clinical utility related to 18F-FDG PET/CT. To measure clinical utility, NOPR required physicians to complete questionnaires assessing the indication for imaging as well as pre- and post-PET treatment plans. In a 2008 study from NOPR incorporating data from 40 863 18F-FDG studies performed at 1368 centres, it was reported that 38% of patients experienced a change in intended management as a result of this imaging test [6]. In light of these and other data from NOPR, 18F-FDG PET/CT is now widely used across a range of tumour histologies. Moreover, this imaging study is readily reimbursed by both the CMS and private insurers.
In summary, we are delighted by the results of Albisinni et al. [3] and look forward to other prospective studies (for example ClinicalTrials.gov identifier NCT02825875) that aim to define the clinical utility of PSMA-targeted PET imaging of prostate cancer.
Article of the Week: Impact of Re-TUR on BCG-Treated T1 HG/G3 Bladder Cancer
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from , discussing their paper.
If you only have time to read one article this week, it should be this one.
The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette–Guerin
Objectives
To determine if a re-transurethral resection (TUR), in the presence or absence of muscle at the first TUR in patients with T1-high grade (HG)/Grade 3 (G3) bladder cancer, makes a difference in recurrence, progression, cancer specific (CSS) and overall survival (OS).
Patients and methods
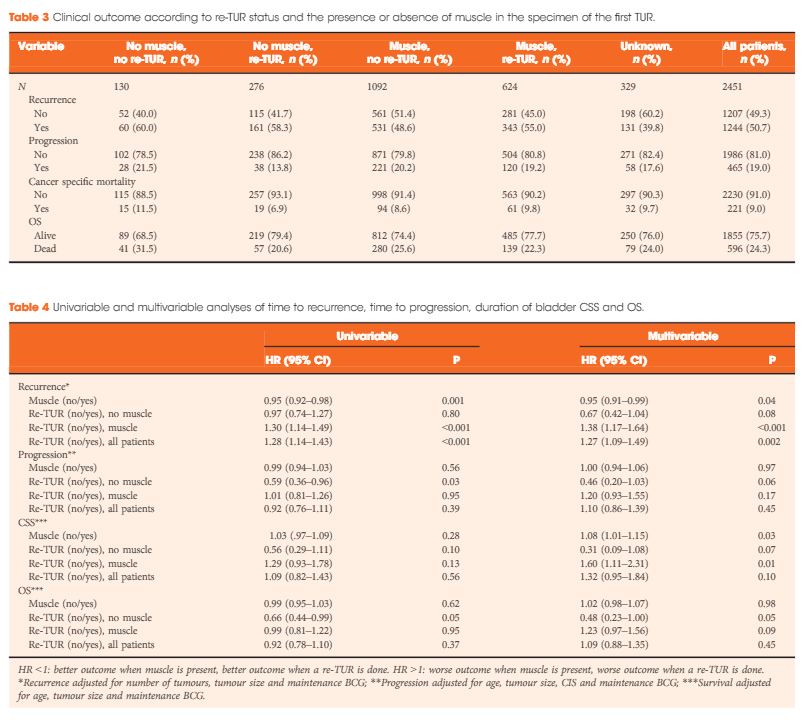
In a large retrospective multicentre cohort of 2451 patients with T1-HG/G3 initially treated with bacille Calmette–Guérin, 935 (38%) had a re-TUR. According to the presence or absence of muscle in the specimen of the primary TUR, patients were divided in four groups: group 1 (no muscle, no re-TUR), group 2 (no muscle, re-TUR), group 3 (muscle, no re-TUR) and group 4 (muscle, re-TUR). Clinical outcomes were compared across the four groups.
Results
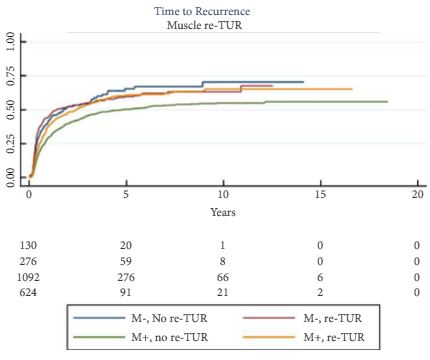
Re-TUR had a positive impact on recurrence, progression, CSS and OS only if muscle was not present in the primary TUR specimen. Adjusting for the most important prognostic factors, re-TUR in the absence of muscle had a borderline significant effect on time to recurrence [hazard ratio (HR) 0.67, P = 0.08], progression (HR 0.46, P = 0.06), CSS (HR 0.31, P = 0.07) and OS (HR 0.48, P = 0.05). Re-TUR in the presence of muscle in the primary TUR specimen did not improve the outcome for any of the endpoints.
Conclusions
Our retrospective analysis suggests that re-TUR may not be necessary in patients with T1-HG/G3, if muscle is present in the specimen of the primary TUR.
Editorial: Time to re-evaluate and refine re-TUR in bladder cancer?
In this issue of BJUI, Gontero et al. [1] present data from a large multi-centre study that should allow us to re-evaluate and refine the indications for re-transurethral resection (TUR) in bladder cancer.
Herr [2] first described this procedure in 1999 and for the past 16 years the indications have remained largely unchanged and are summarised in the latest European Association of Urology guidelines on non-muscle-invasive bladder cancer (NMIBC) [3]:
- After incomplete initial TUR of bladder tumour (TURBT).
- If there is no muscle in the specimen after initial resection.
- In all T1 tumours.
- In all Grade 3 tumours except primary carcinoma in situ.
In a multi-centre retrospective study of 2 451 patients with high-grade (HG)/Grade 3 (G3) T1 NMIBC treated with BCG, Gontero et al. [1]examined 935 patients who had re-TUR (38% of the total, itself a low figure). Patients were divided into four groups according to the presence or absence of detrusor muscle in the first TURBT specimen:
- No muscle, no re-TUR
- No muscle, re-TUR
- Muscle, no re-TUR
- Muscle, re-TUR
The authors found that re-TUR only had a positive impact on recurrence, progression, cancer-specific and overall survival, if detrusor muscle was not present in the original specimen. Importantly, in the presence of detrusor muscle in the original specimen, re-TUR did not improve outcomes. The authors conclude that re-TUR may be unnecessary in HG/G3 T1 patients if detrusor muscle is present at the first TURBT.
These findings are important for two reasons: firstly, Herr’s [2] paper was the first to draw attention to the finding that TURBT, a routine urological procedure, was often carried out inadequately. In recent years, the importance of carrying out a high-quality TURBT has been increasingly recognised [4], whilst the presence of detrusor muscle in the TURBT specimen has been shown to be a good measure of the technical quality of a TURBT [5]. This paper [1] further reinforces the importance of obtaining detrusor muscle in the first TURBT. Indeed, as failure to do so results in the patient having to have a second operation and delays their treatment, perhaps we should start to think of a failure to obtain detrusor muscle at the first TURBT in much the same way as positive margin rates are used as a measure of the quality of radical prostatectomy and by inference, the skill of the surgeon.
Secondly, re-TUR arguably serves one overarching purpose: to identify patients with muscle-invasive bladder cancer (MIBC) who have been under-staged by an inadequate first TURBT and who without a re-TUR would be inadequately treated.
Although a secondary role of re-TUR is to identify patients with residual NMIBC, which has some prognostic value, in practice it rarely changes the patient’s management in this setting, which is intravesical therapy usually with BCG. However, in many healthcare systems the timely organisation of a re-TUR within the recommended 6 weeks is challenging and there is usually a further delay of at least 2 weeks until the pathology is reviewed and a patient with NMIBC can finally commence treatment. In this context, it is not surprising that a recent paper in BJUI showed that the interval to re-TUR was a predictor of recurrence and progression and that a re-TUR after 7 weeks was associated with a much worse outcome [6]. It therefore seems logical to reserve re-TUR only for those patients who truly need it, so that limited resources are focused on ensuring that they receive their operation in a timely manner, ideally within 2–4 weeks. If adopted into day-to-day urological practice, the findings by Gontero et al. [1] will allow many patients with HG/G3 T1 and detrusor muscle in the first TURBT specimen to avoid a re-TUR and start intravesical therapy without further delay. Pragmatically, the same should apply to patients with HG/G3 Ta with detrusor muscle in the specimen. On the other hand, HG/G3 T1 patients without detrusor muscle should be fast-tracked for re-TUR as soon as is practicable and certainly no later than 6 weeks.
The article [1] does have some shortcomings. The study design excludes patients with MIBC, so we do not know by comparison how many patients with MIBC were under-staged at the initial TUR based on subsequent re-TUR but as the authors point out, their conclusions would hold true even in this group, as it is very unlikely that one would miss MIBC if there was adequate detrusor muscle in the pathology specimen.
In conclusion, we should consider refining the indications for re-TUR to improve the utilisation of healthcare resources and ensure that for those that need it, a re-TUR is carried promptly whilst for those that do not, essential intravesical treatment is not delayed.
Video: Impact of Re-TUR on BCG-Treated T1 HG/G3 Bladder Cancer
The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette–Guerin
Objectives
To determine if a re-transurethral resection (TUR), in the presence or absence of muscle at the first TUR in patients with T1-high grade (HG)/Grade 3 (G3) bladder cancer, makes a difference in recurrence, progression, cancer specific (CSS) and overall survival (OS).
Patients and methods
In a large retrospective multicentre cohort of 2451 patients with T1-HG/G3 initially treated with bacille Calmette–Guérin, 935 (38%) had a re-TUR. According to the presence or absence of muscle in the specimen of the primary TUR, patients were divided in four groups: group 1 (no muscle, no re-TUR), group 2 (no muscle, re-TUR), group 3 (muscle, no re-TUR) and group 4 (muscle, re-TUR). Clinical outcomes were compared across the four groups.
Results
Re-TUR had a positive impact on recurrence, progression, CSS and OS only if muscle was not present in the primary TUR specimen. Adjusting for the most important prognostic factors, re-TUR in the absence of muscle had a borderline significant effect on time to recurrence [hazard ratio (HR) 0.67, P = 0.08], progression (HR 0.46, P = 0.06), CSS (HR 0.31, P = 0.07) and OS (HR 0.48, P = 0.05). Re-TUR in the presence of muscle in the primary TUR specimen did not improve the outcome for any of the endpoints.
Conclusions
Our retrospective analysis suggests that re-TUR may not be necessary in patients with T1-HG/G3, if muscle is present in the specimen of the primary TUR.
Article of the Month: Choline-PET/CT radical PCa treatment
Every Month the Editor-in-Chief selects the Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Clinical utility of 18F-fluorocholine positron-emission tomography/computed tomography (PET/CT) in biochemical relapse of prostate cancer after radical treatment: results of a multicentre study
OBJECTIVE
To evaluate 18F-fluorocholine positron-emission tomography (PET)/computed tomography (CT) in restaging patients with a history of prostate adenocarcinoma who have biochemical relapse after early radical treatment, and to correlate the technique’s disease detection rate with a set of variables and clinical and pathological parameters.
PATIENTS AND METHODS
This was a retrospective multicentre study that included 374 patients referred for choline-PET/CT who had biochemical relapse. In all, 233 patients who met the following inclusion criteria were analysed: diagnosis of prostate cancer; early radical treatment; biochemical relapse; main clinical and pathological variables; and clinical, pathological and imaging data needed to validate the results. Criteria used to validate the PET/CT: findings from other imaging techniques, clinical follow-up, treatment response and histological analysis. Different statistical tests were used depending on the distribution of the data to correlate the results of the choline-PET/CT with qualitative [T stage, N stage, early radical prostatectomy (RP) vs other treatments, hormone therapy concomitant to choline-PET/CT] and quantitative [age, Gleason score, prostate-specific antigen (PSA) levels at diagnosis, PSA nadir, PSA level on the day of the choline-PET/CT (Trigger PSA) and PSA doubling time (PSADT)] variables. We analysed whether there were independent predictive factors associated with positive PET/CT results.
RESULTS
Choline-PET/CT was positive in 111 of 233 patients (detection rate 47.6%) and negative in 122 (52.4%). Disease locations: prostate or prostate bed in 26 patients (23.4%); regional and/or distant lymph nodes in 52 (46.8%); and metastatic bone disease in 33 (29.7%). Positive findings were validated by: results from other imaging techniques in 35 patients (15.0%); at least 6 months of clinical follow-up in 136 (58.4%); treatment response in 24 (10.3%); histological analysis of lesions in 17 (7.3%); and follow-up plus imaging results in 21 (9.0%). The statistical analysis of qualitative variables, corresponding to patients’ clinical characteristics, and the positive/negative final PET/CT results revealed that only whether or not early treatment with RP was done was statistically significant (P < 0.001), with the number of positive results higher in patients who did not undergo a RP. Among the quantitative variables, Gleason score, Trigger PSA and PSADT clearly differentiated the two patient groups (positive and negative choline-PET/CT: P = 0.010, P = 0.001 and P = 0.025, respectively). A Gleason score of <5 or ≥8 clearly differentiated positive from negative PET. Trigger PSA: mean of 8 ng/mL for positive PET/CT vs 2.8 ng/mL for negative PET/CT; PSADT: mean of 8 months for positive vs 12.6 months for negative. The optimal threshold values were: 3 ng/mL for Trigger PSA level and 6 months for PSADT (Youden index/receiver operating characteristic curve). Analysing these two variables together showed that PSADT was more conclusive in patients with lower Trigger PSA levels. Analysing variables by location showed that only PSADT was able to differentiate between those with disease confined to the prostate compared with the other two locations (lymph nodes and bone), with shorter PSADT in these two, which was statistically significant (P < 0.002). In the patient group with a PSA level of <1.5 ng/mL, 30.8% had the disease, 7% of whom had metastatic bone disease. In the multivariate logistic regression, the risks factors that were clearly independent for those with positive PET/CT were: PSA level of >3 ng/mL, no early RP, and Gleason score of ≥8.
CONCLUSIONS
Our results support the usefulness of 18F-fluorocholine PET/CT in biochemical relapse of prostate cancer after radical treatment, with an overall disease detection rate close to 50%, and it can be recommended as first-line treatment. As mentioned above, besides Trigger PSA levels, there are other clinical and pathological variables that need to be considered so as to screen patients properly and thus minimise the number of nodular lesions and increase the diagnostic accuracy of the examination.
Editorial: Choline-PET/CT in relapsing prostate cancer patients
18F-choline positron emission tomography (PET)/C T has become a modern imaging technique in men with prostate cancer and biochemical relapse after local treatment with curative intent (radical prostatectomy, external beam/intensity-modulated radiation therapy, brachytherapy) in order to differentiate between local, locoregional and systemic relapse. Although 18F-choline PET/CT will probably be replaced by prostate-specific membrane antigen-PET/CT in the near future, the present paper by Rodada-Marina et al. [1] is important for daily routine because the authors attempt to define the current role of 18F-choline PET/CT in the diagnostic algorithm of men with relapsing PSA and to define specific patient cohorts in whom 18F-choline PET/CT might have a significant impact in the decision-making process regarding the most appropriate treatment.
Two issues are important to me when discussing the potential indication for performing new imaging studies in my patients with relapsing PSA: (1) whether the method is sensitive enough to detect a metastatic deposit at a given PSA serum concentration and (2) whether a positive finding using this imaging method would change my treatment recommendation. In this context, the current recommendation is 18F-choline PET/CT at a PSA serum concentration >1 ng/mL if a therapeutic consequence will be drawn [2]. If the patient would not be a candidate for a secondary local treatment option, such as salvage radiation therapy or salvage radical prostatectomy, but he would be treated with androgen deprivation therapy anyhow, none of the modern imaging studies would make sense.
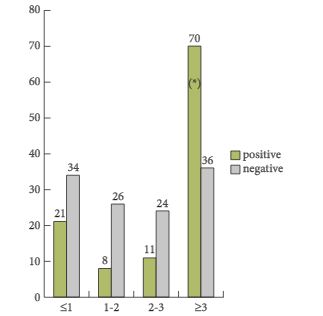
In the present paper, a total of 233 patients from six different institutions were included in a retrospective study. One of the most important findings of this paper is that the detection rate was only 47.6%, despite relatively high mean and median trigger PSA serum levels of 5.3 and 2.8 ng/mL, respectively. The detection rates varied between 23.5 and 38.2% in men with PSA serum levels between <1 and 2–3 ng/mL and the detection only increased to 67% in men with PSA levels ≥3 ng/mL. Moreover, the authors identified that the best threshold for the trigger PSA level was 3.5 ng/mL, with a sensitivity and a specificity of 64 and 76%, respectively. With regard to PSA doubling time (PSA-DT), the best threshold was < 6 months, with a sensitivity and a specificity of 58% only. Based on these very high PSA serum levels at the time of imaging studies, which had the potential intent to select the most appropriate therapy, the majority of patients were already beyond the scope of secondary local therapy with curative intent [2, 3]. Furthermore, it was shown that patients with a Gleason score 8–10 and a PSA-DT of <6 months have a higher probability of having systemic disease – a fact which is well known already.
What do these data mean for clinical practice? There might be three clinical scenarios in which imaging studies might exert a significant impact on further treatment: (1) salvage radiation therapy in men with PSA relapse after radical prostatectomy (RP) [2, 3], (2) salvage RP after radiation therapy of the prostate [4] and (3) salvage pelvic lymphadenectomy in men with PSA relapse after RP or radiation therapy of the prostate [5]. In my view, the data underline the fact that imaging with 18F-choline PET/CT is not helpful in the first clinical scenario, early or late PSA relapse after RP. The clinician needs to start local salvage therapy, such as percutaneous radiation therapy, at a serum PSA concentration well below 0.5 ng/mL if a curative intent is the focus of treatment [2, 3]. Based on the current data, only one fifth of the patient cohort had a positive 18F-choline PET/CT finding even when considering aggressive biological features such as a high Gleason score, a rapid PSA-DT and a high PSA nadir after RP; therefore, PET/CT does not add significant additional diagnostic information in the individual patient so that it does not appear useful to perform 18F-choline PET/CT in men with low PSA levels at time of relapse. 18F-choline PET/CT might be helpful in the second clinical scenario to identify patients who will benefit from salvage RP. It has been shown that a PSA < 10 ng/mL and a PSA-DT >12 months at time of surgery are the most significant prognosticators for identifying organ-confined disease [4]. A positive detection rate for metastatic foci would be >75% in this scenario, underlining the indication for performing choline PET/CT. With regard to the third clinical scenario, it has been shown that a serum PSA <4 ng/mL and a slow PSA-DT represent prognostic markers for selecting men who most probably have locoregional relapse in the small pelvis and who will benefit the most from salvage lymphadenectomy [5]. Again, choline-PET/CT is indicated to exclude retroperitoneal or systemic disease and it should be performed before any salvage procedure.
In conclusion, the retrospective study performed by Rodado-Marina et al. [1] provides significant and clinically useful information with regard to the definition of a patient cohort that would benefit most from the performance of a choline PET/CT. This information should be considered when counselling patients with regard to the need for new imaging methods at the time of PSA relapse.
Article of the Week: Assessing extranodal extension and the size of the largest lymph node metastasis after RP
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Prognosis of patients with pelvic lymph node metastasis following radical prostatectomy: value of extranodal extension and size of the largest lymph node metastasis
Niccolo M. Passoni, Harun Fajkovic*, Evanguelos Xylinas†, Luis Kluth‡, Christian Seitz*, Brian D. Robinson§, Morgan Rouprêt¶, Felix K. Chun‡, Yair Lotan**, Claus G. Roehrborn**, Joseph J. Crivelli§, Pierre I. Karakiewicz††, Douglas S. Scherr§, Michael Rink‡, Markus Graefen‡, Paul Schramek*, Alberto Briganti, Francesco Montorsi, Ashutosh Tewari§ and Shahrokh F. Shariat*§**
Department of Urology, Urological Research Institute, University Vita-Salute San Raffaele, Milan, Italy, *Department of Urology, Medical University of Vienna, Vienna, Austria, †Department of Urology, Cochin Hospital, Assistance Publique-Hôpitaux de Paris, University Paris Descartes, ¶Academic Department of Urology of la Pitié-Salpêtrière, Assistance Publique-Hôpitaux de Paris, Faculté de medicine Pierre et Marie Curie, University Paris VI, Paris, France, ‡Medical Centre Hamburg-Eppendorf, Martini Clinic, Prostate Cancer Center at University Medical Center Hamburg-Eppendorf, Hamburg, Germany, §Department of Urology and Pathology, Weill Cornell Medical College, New York Presbyterian Hospital, New York, NY, **Department of Urology, Southwestern Medical Center, University of Texas, Dallas, TX, USA, and ††Department of Urology, University of Montreal, Montreal, QC, Canada
OBJECTIVE
- To assess the prognostic role of extranodal extension (ENE) and the size of the largest lymph node (LN) metastasis in predicting early biochemical relapse (eBCR) in patients with LN metastasis after radical prostatectomy (RP).
PATIENTS AND METHODS
- We evaluated BCR-free survival in men with LN metastases after RP and pelvic LN dissection performed in six high-volume centres.
- Multivariable Cox regression tested the role of ENE and diameter of largest LN metastasis in predicting eBCR after adjusting for clinicopathological variables.
- We compared the discrimination of multivariable models including ENE, the size of largest LN metastasis and the number of positive LNs.
RESULTS
- Overall, 484 patients were included. The median (interquartile range, IQR) follow-up was 16.1 (6–27.5) months. The median (IQR) number of removed LNs was 10 (4–14), and the median (IQR) number of positive LNs was 1 (1–2).
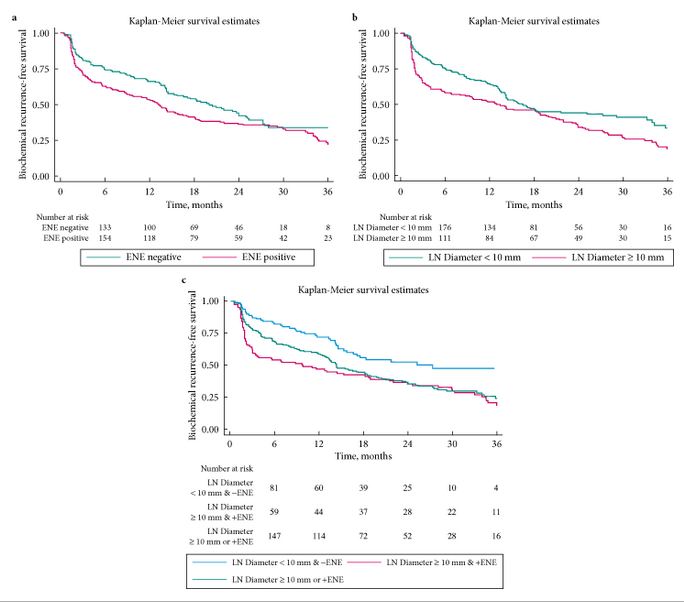
- ENE was present in 280 (58%) patients, and 211 (44%) had their largest metastasis >10 mm. Patients with ENE and/or largest metastasis of >10 mm had significantly worse eBCR-free survival (all P < 0.01).
- On multivariable analysis, number of positive LNs (≤2 vs >2) and the diameter of LN metastasis (≤10 vs >10 mm), but not ENE, were significant predictors of eBCR (all P < 0.003).
- ENE and diameter of LN metastasis increased the area under the curve of a baseline multivariable model (0.663) by 0.016 points.
CONCLUSIONS
- The diameter of the largest LN metastasis and the number of positive LNs are independent predictors of eBCR.
- Considered together, ENE and the diameter of the largest LN metastasis have less discrimination than the number of positive LNs.