Editorial: The new frontier of prostate biopsy: determining the role of image-guidance in moving the needle
One of the most pressing topics in urological oncology concerns the role of MRI/ultrasonography (US)-fusion guided biopsies in detecting prostate cancer. The literature on this emerging technology is permeated by questions regarding when it should be used, how it should be performed, and which patients stand to benefit. The stakes are high to figure this out, as real patients will continue to suffer from missteps made in diagnosis and treatment of prostate cancer whilst we seek to improve our detection methods.
In this issue of BJUI, Borkowetz et al. [1] compare prostate cancer detection rates between MRI/US-fusion targeted and conventional systematic biopsies. They prospectively enrolled a cohort of biopsy-naïve men who had an elevated PSA level and/or an abnormal DRE. All men received both a transperineal MRI/US-fusion biopsy and a systematic TRUS-guided biopsy. They found that combining both approaches led to improved detection rates for clinically significant and overall prostate cancer compared to either method alone.
A strength of this study [1] is its focus on biopsy-naïve men, for which data on the comparison of biopsy techniques is relatively limited. Siddiqui et al. [2] were instrumental in demonstrating that targeted MRI/US-fusion biopsy, compared to the systematic TRUS biopsy, was associated with improved diagnostic accuracy for higher-risk tumours and decreased detection of low-risk disease. However, the large majority of men in the study had received a prior biopsy, making it difficult to generalise the results to biopsy-naïve men. Focusing on biopsy-naïve men is of great importance – men referred for a biopsy after an elevated PSA level or abnormal DRE increasingly face conflicting opinions about the next best step in diagnostic evaluation, especially given the recent influx of imaging and biomarker tests that aim to guide this decision point.
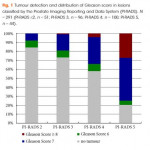
One group previously compared MRI-guided and systematic TRUS biopsy in a large group of biopsy-naïve of men and found the MRI-guided in-bore technique to be superior in detecting significant cancers and avoiding insignificant cancers [3]. However, that study was limited by both its definition of ‘significant cancer’ (it included Gleason 3 + 3 disease) and the variance in the definition of ‘low-risk’ cancer between MRI-guided and TRUS biopsies. By contrast, the present authors uniformly defined significant cancer as Gleason ≥3 + 4. This is just one example highlighting the widespread disagreement over the parameters used to measure the efficacy of targeted-biopsy techniques in cancer detection. Continued incorporation of standardised scales such as the Prostate Imaging Reporting and Data System (PI-RADS), which was also used in the accompanying article, will help to mitigate some of this disagreement in future studies. Of course, these standardised scales are still subject to inter- and intra-observer variability, which may decrease with further clarification of the grading systems, as well as appropriate reader training [4].
Whilst Borkowetz et al. [1] helped to fill important gaps in the literature, their study was not without limitations. They included PI-RADS 2 scores for targeted biopsies although many urologists would consider these lesions insignificant and not worth targeting. Furthermore, comparing a transperineal MRI/US-fusion with a transrectal systematic approach makes it difficult to separate the true effect of the targeted approach from that of the anatomical approach. Using both methods in each patient also precludes any comparison of complication rates between the biopsy approaches, an important clinical endpoint for both the urologists administering the biopsies and the patients enduring the complications.
Methodology aside, the new frontier of prostate biopsy technique still relies on a basic triad of efficacy: (i) improved accuracy of cancer detection, (ii) reduced complication rates, and (iii) manageable cost and practicality of widespread implementation. For the first tenet, the recently published PROstate MRI Imaging Study (PROMIS) trial cemented the ability of multi-parametric MRI to detect clinically significant prostate cancer with greatly improved sensitivity and negative predictive value over TRUS [5]. The ongoing randomised PRostate Evaluation for Clinically Important Disease: Sampling Using Image-guidance Or Not? (PRECISION) trial will hopefully add to the step taken by Borkowetz et al. [1], by comparing MRI-targeted and systematic TRUS biopsies on the outcomes of accuracy in cancer detection, adverse events, patient health-related quality of life, and cost [6]. Continued investigation in all of these areas will be crucial to guiding how we move the needle in prostate cancer diagnostics.