Editorial: Translating cost-utility modelling into the real world – the case of focal high-intensity focussed ultrasound and active surveillance
Health economic modelling is always a challenge. The inputs are never quite what we want them to be. The literature that we have at our disposal suffers from the inevitable deficiencies of lack of maturity, ever diminishing relevance, and questionable applicability as practice evolves. The modelling can never quite reflect the nuances and vagaries of clinical practice. However, the process is an important and in some cases (evaluation by the UK’s National Institute of Clinical and Care Excellence) a necessary one. Knowing the cost of achieving a given health status over a defined time frame is an important consideration in the allocation resource in any finite system of care.
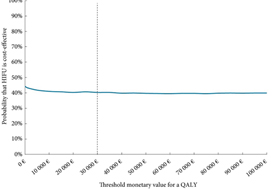
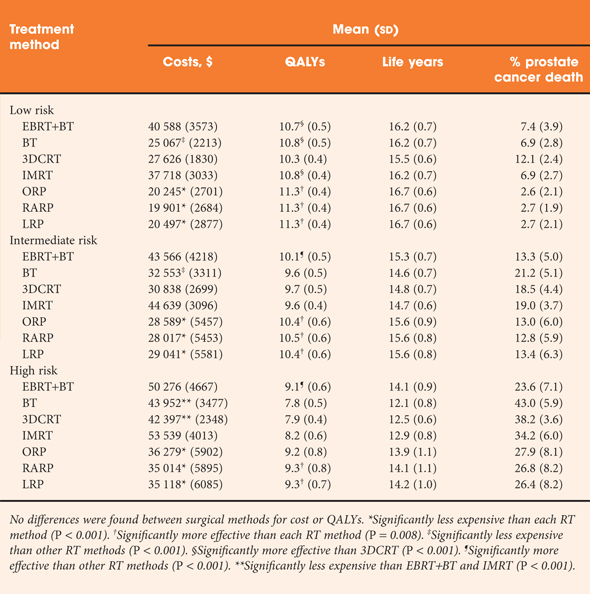
The paper by Bénard et al. [1] is most useful in helping us to understand what the issues are and how our decision-making might impact on cost in the context of low-to-moderate risk prostate cancer.
The issue with these types of analyses is the degree to which the inevitable assumptions made by the investigators are consistent with current practice. Below I have tried to identify some of the areas in which the assumptions diverge from current knowledge and ‘know-how’, in order to illustrate just how difficult the task that Bénard et al. [1] have undertaken.
The first relates to the assumption that both strategies can be applied to the same population. They cannot, or perhaps more correctly – should not. For instance, nobody I know would offer a man focal treatment who had well-characterised micro-focal low-volume Gleason 3+3 (or Gleason Grade Group 1) [2]. We know, from what now constitutes a considerable body of level-1 evidence, that there is no benefit to be derived from intervening in disease that confers little, if any, risk of premature death [3]. Today, focal therapy tends to be applied to men with well-characterised, visually localised Gleason Grade Group ≥2, who want to avoid radical whole gland therapy and the genitourinary side-effects associated with them [4].
The second relates to the synergies between the two treatments. Increasingly men who opt for active surveillance (AS) upfront have an increasing tendency to opt for focal treatment on radiological progression of any lesion under scrutiny. This makes quite a bit of intuitive sense. These are men who appear comfortable with the process of observation, are likely to place high utility on genitourinary function, may have exhibited a very stable background prostate (apart from the expanding lesion depicted on MRI), are likely to be very well informed, and will, by now, be very well-characterised histologically. These, as it happens, are the ideal attributes for a candidate for focal therapy.
The third is a reflection on the relevance of the literature to inform the question being posed. It is no fault of the authors that AS has changed beyond recognition in the last few years. This change has been driven by the use of MRI in the risk stratification process for candidate selection, the substation of temporal biopsy assessment by imaging and the reduction, and at times elimination, of the re-classification vs progression error that confounds most of the literature on
surveillance. Modelling events on historical single-institution cohorts (as AS has never been evaluated in a randomised setting apart from one comparison against focal therapy) is probably unhelpful in helping us to understand and inform our future [5].
The fourth concerns scope. Why limit this analysis to focal high-intensity focussed ultrasound? All focal therapies, irrespective of energy source, seem to produce very similar outcomes, both in terms of freedom from failure (time to radical treatment and/or metastasis) and in relation to preservation of genitourinary function. Broadening the scope, by including vascular targeted photo-therapy and cryotherapy, would have meant that randomised trials could have been
included as inputs, with the effect of possibly reducing the high levels of uncertainty that bedevil the current analysis [5,6].
The fifth recognises the dynamic nature of the progression risk in AS cohorts. This is an important, but poorly recognised, attribute of the mature AS cohorts that we tend to rely upon. These cohorts are dynamic entities that have as entrants men of increasingly lower risk (due to a recent improvement in risk stratification) and, at the same time, continually exit the very men with the highest risk, i.e., the ‘progressors’. Thus, over time, the cohort undergoes a gradual, but inevitable, reduction in risk. The more mature the cohort, the greater the reduction. By referencing mature cohorts (when trying to predict the fate of future patients) we
will, therefore, have a tendency to over-estimate the benefit/safety of AS in a contemporary setting.
This is not to say that we should not endeavour to estimate the cost of achieving a given health state. We need this, perhaps more than ever. What we need to strive towards are models that represent both the reality of practice and the very latest, and most subtle, distillation of the current evidence.
by Mark Emberton
References
- Bénard A, Duroux T, Robert G. Cost-utility analysis of focal high-intensity focussed ultrasound vs active surveillance for low- to intermediate-risk prostate cancer using a Markov multi-state model. BJU Int 2019; 124: 962–71
- Klotz L, Emberton M. Management of low risk prostate cancer-active surveillance and focal therapy. Nat Rev Clin Oncol 2014; 11: 324–34
- Hamdy FC, Donovan JL, Lane JA et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–24
- Elliott D, Hamdy FC, Leslie TA et al. Overcoming difficulties with equipoise to enable recruitment to a randomised controlled trial of partial ablation vs radical prostatectomy for unilateral localised prostate cancer. JU Int 2018; 122: 970–7
- Azzouzi AR, Vincendeau S, Barret E et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 2017; 18: 181–91
- Donnelly BJ, Saliken JC, Brasher PM et al. A randomized trial of external beam radiotherapy versus cryoablation in patients with localized prostate cancer. Cancer 2010; 116: 323–30