Article of the Week: A mpMRI-based risk model to determine the risk of prostate cancer prior to biopsy
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy
Abstract
Objective
To develop and externally validate a predictive model for detection of significant prostate cancer.
Patients and Methods
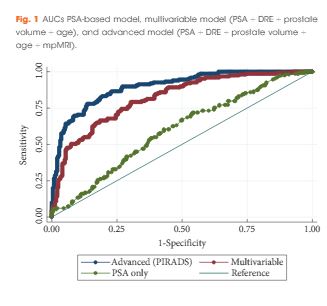
Development of the model was based on a prosp ctive cohort including 393 men who underwent multiparametric magnetic resonance imaging (mpMRI) before biopsy. External validity of the model was then examined retrospectively in 198 men from a separate institution whom underwent mpMRI followed by biopsy for abnormal prostate-specific antigen (PSA) level or digital rectal examination (DRE). A model was developed with age, PSA level, DRE, prostate volume, previous biopsy, and Prostate Imaging Reporting and Data System (PIRADS) score, as predictors for significant prostate cancer (Gleason 7 with >5% grade 4, ≥20% cores positive or ≥7 mm of cancer in any core). Probability was studied via logistic regression. Discriminatory performance was quantified by concordance statistics and internally validated with bootstrap resampling.
Results
In all, 393 men had complete data and 149 (37.9%) had significant prostate cancer. While the variable model had good accuracy in predicting significant prostate cancer, area under the curve (AUC) of 0.80, the advanced model (incorporating mpMRI) had a significantly higher AUC of 0.88 (P < 0.001). The model was well calibrated in internal and external validation. Decision analysis showed that use of the advanced model in practice would improve biopsy outcome predictions. Clinical application of the model would reduce 28% of biopsies, whilst missing 2.6% significant prostate cancer.
Conclusions
Individualised risk assessment of significant prostate cancer using a predictive model that incorporates mpMRI PIRADS score and clinical data allows a considerable reduction in unnecessary biopsies and reduction of the risk of over-detection of insignificant prostate cancer at the cost of a very small increase in the number of significant cancers missed.