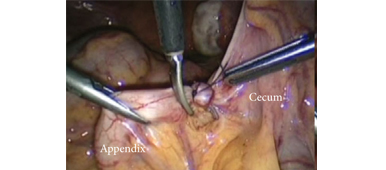
Here, we present a rare case in which a urothelial carcinoma was located at the lower moiety of a complete duplex right kidney.
Authors: PEI-YU LIN1, VICTOR CHIA-HSIANG LIN1,2, RENG-HONG WU3, TSAN-JUNG YU1,2
Department of Urology 1, E-Da Hospital/I-Shou University, Kaohsiung City 2, Department of Radiology3, Chi-Mei Medical Center, Tainan City, Taiwan
Corresponding Author: Victor Chia-Hsiang Lin, M.D., Division of Urology, Department of Surgery, Minimally Invasive Surgical Center, E-Da Hospital/I-Shou University, Kaohsiung City 824, Taiwan 824. E-mail: [email protected]
Abstract
Total nephroureterectomy is usually considered to be the gold standard treatment for urothelial carcinoma involving the kidney, where nephron sparing surgery is seldom considered because of the concerns of tumor spillage. However, heminephrectomy with ipsilateral ureterectomy may be performed when the malignancy occurs in a patient with a renal fusion anomaly or in a single moiety of a complete duplex kidney. Here, we present a unique case with urothelial carcinoma in the lower moiety of a complete duplex right kidney. Laparoscopic right heminephrectomy with ureterectomy was performed, after preoperative evaluation with reconstructed 3-dimensional computed tomography angiography and intraoperative navigation with laparoscopic ultrasound.
Complete duplication of the collecting system is an uncommon congenital anomaly, occurring in approximately 1 in 125 individuals. Duplex kidneys can be associated with ectopic ureters, ureteroceles, and vesicoureteric reflux, and cause various clinical manifestations including incontinence, voiding dysfunction and urinary tract infection. A common disease variant in this entity is a ectopic ureteric orifice, associated with a dysplastic poorly functioning upper-pole renal moiety. Heminephrectomy is considered to be the treatment of choice for patients with certain pathologies. Here, we present a rare case in which a urothelial carcinoma was located at the lower moiety of a complete duplex right kidney. The patient underwent laparoscopic heminephrectomy with ureterectomy after thorough preoperative evaluation.
Case Report
An 82-year-old female presented with the chief complaint of intermittent painless gross hematuria for 3 months. Physical examination was unremarkable. Cystoscopy with retrograde pyelography revealed complete duplication of the right collecting system and a 2×2 cm filling defect located over the lower moiety of the duplex right kidney (Fig. 1 A & B).
Figure 1. Retrograde pyelography revealed duplication of the right collecting system. A. Hydronephrosis of the right upper moiety due to external compression indicated by a black arrow. B. The white arrow indicates a filling defect over the lower calyx of the right duplex kidney.
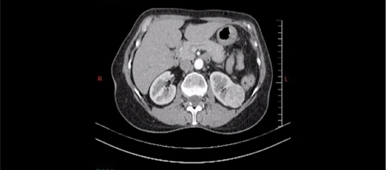
Reconstructed 3-dimensional computed tomography (3-D CT) angiography demonstrated a tumor in the lower moiety renal pelvis of the right duplex kidney and several parapelvic renal cysts compressing the upper moiety, causing hydronephrosis and hydrocalycosis. (Fig. 2 A & B).
Figure 2. A. Coronal view of a computed tomography (CT) scan showing a tumor in the duplex right renal pelvis as indicated by the white arrow. B. Reconstruction CT clearly demonstrated a soft tissue mass over the lower calyx of the duplex right kidney, as indicated by the white arrow.
After discussing the available options with the patient and her family, they agreed for her to undergo laparoscopic right heminephrectomy and ureterectomy.
Diagnostic ureteroscopy showed a large nodular tumor in the lower moiety of the duplex right kidney. Subsequently, a transperitoneal laparoscopic technique was adopted, taking down the mesocolon of the ascending colon from the hepatic flexure to the level of the bifurcation of the right iliac vessels. After deroofing the parapelvic renal cysts, the right renal hilum was carefully exposed and the supplying branches to the lower moiety were skeletonized individually. The arterial branch which supplied the lower moiety was confirmed and clamped using a surgical bulldog clamp. The lower moiety appeared cyanotic when its supplying branch was clamped correctly. The venous branch of the lower moiety was accompanied by the arterial branch and was positively identified. By first clipping and dividing the branches of the right renal artery supplying the lower moiety, a clear line of demarcation between the ischaemic lower moiety and the normal upper moiety was seen. Laparoscopic ultrasound further confirmed the upper margin of the lower moiety calyces, and cutting and division of the moieties were performed precisely and efficiently with electrocautery and ultrasonic shears. Hemostasis of the bare surface of the upper moiety was achieved with laparoscopic free-hand suture and knot-tying. The ureter of the lower moiety was skeletonized to the level of the right iliac vessels, and clips were put on the distal end to prevent extravasation of tumor cells. The specimen was then placed in an endobag. The extravesical bladder cuff excision was performed using an open approach through a right Gibson’s incision. When dissecting into the retroperitoneum, the distal ureter was identified, the endobag in which the lower moiety of the right kidney and its proximal ureteral segment were placed was removed thereafter. Retracting the distal ureter cephalad, dissection was continued distally until the bladder was reached. The bladder was incised just anterior to the ureter and the ureteric lumen was identified. The posterior portion of the ureter was excised and detached from the bladder cuff carefully under vision. The bladder defect was sutured with 1-0 chromic gut. There was no injury to the ureteric orifice of the upper moiety of the right kidney.
The patient’s postoperative recovery was prompt due to her undergoing minimally invasive surgery, and she resumed oral intake 12 hours postoperatively. She received a total amount of meperidine 150mg for postoperative pain relief. She was discharged after two days. Due to a suspected urinary leak, a JJ stent was inserted to drain the right upper moiety on postoperative day 6. Final pathological examination demonstrated urothelial carcinoma of the lower moiety of the right duplex kidney, stage pT1. During two-years of follow-up, no tumor recurrence has been detected.
Discussion
Urothelial carcinoma in duplex kidneys is rare with only sporadic reports in the English literature.1,2 Nephron sparing surgery is well established for the treatment of small exophytic renal cell carcinoma.3 However, total nephroureterectomy is the treatment of choice to manage renal urothelial carcinoma. Nevertheless, nephron sparing surgery for upper tract urothelial carcinoma can still be considered in certain situations such as crossed renal ectopy or tumour in one moiety of a duplex system.4 Gur et al. demonstrated the feasibility of separating the involved kidney from its conjoint to treat patients with transitional cell carcinoma in a fused crossed ectopic kidney. Subsequent ureterectomy with bladder cuff excision was performed in our case with respect to oncological principles. We also advocate the importance of a thorough delineation of the involved renal vasculature using CT-angiography preoperatively.
Similarly, we used reconstructed 3-D CT angiography to delineate the involved renal vasculature. A radiologist also performed laparoscopic ultrasound to help decide precisely the cut-margin during the parenchymal transection. We believe these tools are extremely important in planning this type of surgery. To the best of our knowledge, this is the first case of urothelial carcinoma in one moiety of a complete duplex kidney treated by laparoscopic heminephrectomy and ureterectomy.
In our experience, laparoscopic heminephrectomy is a feasible and safe modality in treating urothelial carcinoma of the lower moiety in a duplex kidney in selected cases.
References
1. Lia-Beng Tan, Biing-Rorn Tserng, Wei-Hwang Huang, Chia-Jiuan Tarn. Synchronous bilateral carcinoma of the ureter in association with unilateral incomplete duplication of the ureter. Urol Int 56: 196-199, 1996.
2. Jenq-Daw Li, Johnny Shinn-Nan Lin, Wei-Jen Yao. Synchronous transitional cell carcinoma in both moieties of an incomplete duplex system. Urology 59: 944-945, 2002.
3. Alireza Moinzadeh, Inderbir S. Gill, Antonio Finelli, Jihad Kaouk and Mihir Desai. Laparoscopic partial nephrectomy: 3-year followup. J Urol 175: 459-462, 2006
4. Uri Gur, Ofer Yossepowitch and Jack Baniel. Transitional cell carcinoma in a fused crossed ectopic kidney. Urology 62: 748, 2003.
Date added to bjui.org: 21/07/2012
DOI: 10.1002/BJUIw-2011-108-web