Article of the Week: Safety and efficacy of 2-weekly cabazitaxel in mCRPC
Every Week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Safety and efficacy of 2-weekly cabazitaxel in metastatic castration-resistant prostate cancer
How to Cite
Clément-Zhao, A., Auvray, M., Aboudagga, H., Blanc-Durand, F., Angelergues, A., Vano, Y. A., Mercier, F., El Awadly, N., Verret, B., Thibault, C. and Oudard, S. (2018), Safety and efficacy of 2-weekly cabazitaxel in metastatic castration-resistant prostate cancer. BJU International, 121: 203–208. doi: 10.1111/bju.13855
Abstract
Objectives
To evaluate the safety and efficacy of a 2-weekly cabazitaxel schedule in patients with metastatic castration-resistant prostate cancer (mCRPC).
Materials and methods
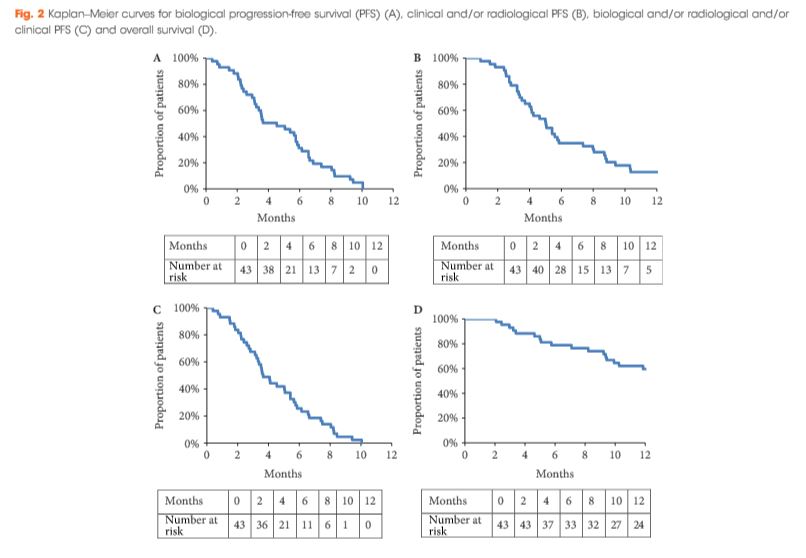
During the period October 2013 to February 2016, 43 patients with mCRPC were treated with cabazitaxel (16 mg/m2, on days 1 and 15 of a 4-week cycle) together with prophylactic granulocyte colony-stimulating factor (G-CSF). The safety profile and efficacy (prostate-specific antigen [PSA] response; biological, clinical or radiological progression-free survival [PFS] and overall survival [OS]) of the treatment were analysed.
Results
All patients had received prior docetaxel and 79.1% abiraterone acetate. At inclusion, 46.5% were aged >70 years and 27.9% had an Eastern Cooperative Oncology Group performance status ≥2. Six patients stopped treatment because of toxicity. Grade ≥3 toxicities were: asthenia (16.3%); neutropenia (11.6%); thrombocytopenia (9.3%); diarrhoea (7%), anaemia (4.7%), febrile neutropenia (4.7%) and haematuria (2.3%). In all, 52.4% achieved a ≥30% PSA response and 40.5% had a ≥50% PSA response. The median OS was 15.2 months.
Conclusion
This prospective pilot study suggests that cabazitaxel 16 mg/m² given 2-weekly has a manageable toxicity profile in docetaxel- and abiraterone acetate-pretreated patients with mCRPC. A prospective phase III trial comparing this regimen with the standard cabazitaxel regimen is planned to confirm these results.