A 36-year old gentleman presented with severe sudden onset unilateral loin pain, suggestive of renal colic. Renal tract ultrasonography was unremarkable, but computerised tomography demonstrated renal infarction. Subsequent angiography proved a renal artery dissection. This uncommon diagnosis should be considered in patients with sudden onset loin pain, particularly those with risk factors such as hypertension, connective tissue disease or trauma.
Authors: King, Chris; Amdekar, Shivan
Corresponding Author: King, Chris
Dr Chris King, MB BChir.
Vascular surgery, Northwick Park Hospital, North West London Hospitals NHS Trust, Watford Road, Harrow, HA1 3UJ
Dr Shivani Amdekar, MB BS
General surgery, Northwick Park Hospital, North West London Hospitals NHS Trust, Watford Road, Harrow, HA1 3UJ
Introduction
Spontaneous renal artery dissection is a rare event, typically constituting only 0.05% of all arteriographic findings on radiological imaging [1]. Diagnosis is typically delayed due to difficulties distinguishing the presentation from more familiar conditions such as renal colic and pyelonephritis. We report the case of a 36-year old office worker presenting with severe loin pain and the 3 days it took to reach a definitive diagnosis.
Case Report
A 36-year old Indian gentleman presented to the emergency department with severe sudden onset right loin pain and vomiting, ongoing for 8-10 hours. The pain came on over a period of minutes, and was exclusive to the flank with no radiation. It was constant in nature, and he had never had a similar pain before. He had no urinary symptoms. The patient was otherwise fit and well. In particular he had no predisposing factors such as atrial fibrillation, hypertension, history of trauma or connective tissue disorder. On examination he was afebrile but diaphoretic, with a tender right flank. There was no evidence of peritonism. Testicular examination was unremarkable. His blood pressure was 152/103 mmHg. He had microscopic haematuria but no nitrites or leukocytes in his urine. His white cell count was 15.8 x109/L and C-reactive protein 2mg/L. His renal function was within normal range (creatinine 93umol/L, urea 4.8mmol/L). A renal ultrasound demonstrated no hydronephrosis or visible ureteric/renal calculi. There was normal renal corticomeduallary differentiation. He was admitted and started on treatment for renal colic. The following night the patient developed tachycardia and after senior review was treated for sepsis. He was started on ciprofloxacin and gentamicin for possible pyelonephritis or retrocaecal appendicitis. An abdominal computerised tomography (CT) scan the next morning demonstrated an acute infarct of the lateral half of the right kidney with accompanying perinephric stranding. CT angiography highlighted a long linear filling defect of the right renal artery in keeping with arterial dissection, completely occluding the anterior branch and partially occluding the posterior branch. All other abdominal arteries were unremarkable. The patient was managed medically with anticoagulation as it was felt that the dissection extended too far distally for any interventional management such as endovascular stenting. Further screens for infective endocarditis, vascultitis and hypercoagulability were negative. His urea and creatinine remained within the normal range throughout this time. The patient’s pain settled and he was discharged from hospital on warfarin and encouraged to resume normal activity. A follow-up magnetic resonance renal angiogram at 6 weeks confirmed dissection as the original cause of infarction, with a subsequent 2.4cm length of completely thrombosed renal artery.
Discussion
There are approximately 200 published cases of spontaneous renal arterial dissection, and it is likely that the condition is underdiagnosed. Most patients are male (a 4:1 ratio), in their 4th-5th decade of life [2]. The condition is associated with hypertension, connective tissue diseases such as Marfan’s and Ehler-Danlos syndromes, fibromuscular dysplasia, atherosclerosis, trauma, and severe physical exertion [3]. The commonest symptom is severe upper abdominal flank pain, often radiating to the epigastrium, but this is not always present [4, 5]. There may be associated nausea and vomiting. One case series of 24 patients reported hypertension in 96% of presentations, gross haematuria in 21% and headaches in 25% [4].
Measurements of serum lactate dehydrogenase may be suggestive of renal infarction [5]. However angiography provides the gold standard for diagnosis [4]. Angiography also allows one to distinguish between causes. Dissections and thromboemboli appear differently on angiography, the former appearing as an abrupt uniform narrowing due to non-filling of the false lumen, whereas the latter display a meniscus crossing the width of the artery [3].
Treatment involves anti-coagulation and control of hypertension, and then either conservative or operative management with the goal of tissue preservation. The latter includes endovascular repair, arterial bypass or nephrectomy [1]. These techniques are best reserved for patients with uncontrolled hypertension, renal insufficiency, or progressive disease as seen on serial angiography [4]. The duration of anticoagulation is largely empirical and based on evidence for carotid dissection [2].
The natural history of the condition is poorly understood due to its rarity and often lack of follow-up. The most common complication is malignant hypertension [4]. Mortality is most associated with renal insufficiency, inherently more common in bilateral disease [4].
Conclusion
Spontaneous renal artery dissection is a rare but important cause of flank pain, and surgeons should be mindful of it as a differential diagnosis. Angiography represents the gold standard investigation. Many patients can be successfully managed conservatively with anti-coagulation and blood pressure control alone, with operative management reserved for those patients with unstable renal functioning or progressive disease.
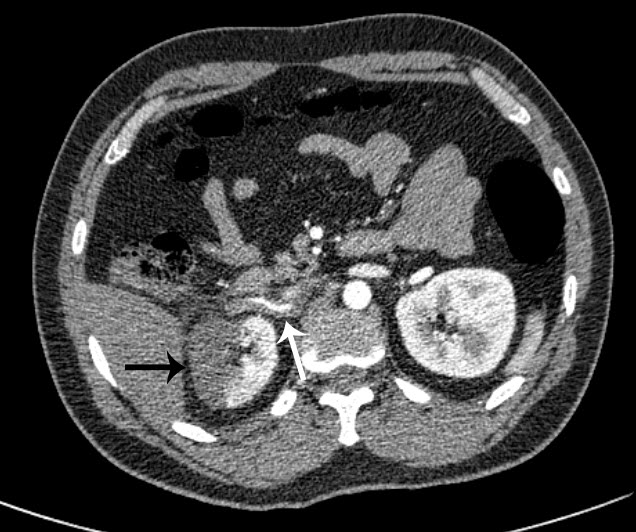 Figure 1 – CT angiography axial image: A long linear filling defect is seen within the right renal artery due to dissection and thrombosis (white arrow). The subsequent renal infarction is evident laterally (black arrow).
Figure 1 – CT angiography axial image: A long linear filling defect is seen within the right renal artery due to dissection and thrombosis (white arrow). The subsequent renal infarction is evident laterally (black arrow).
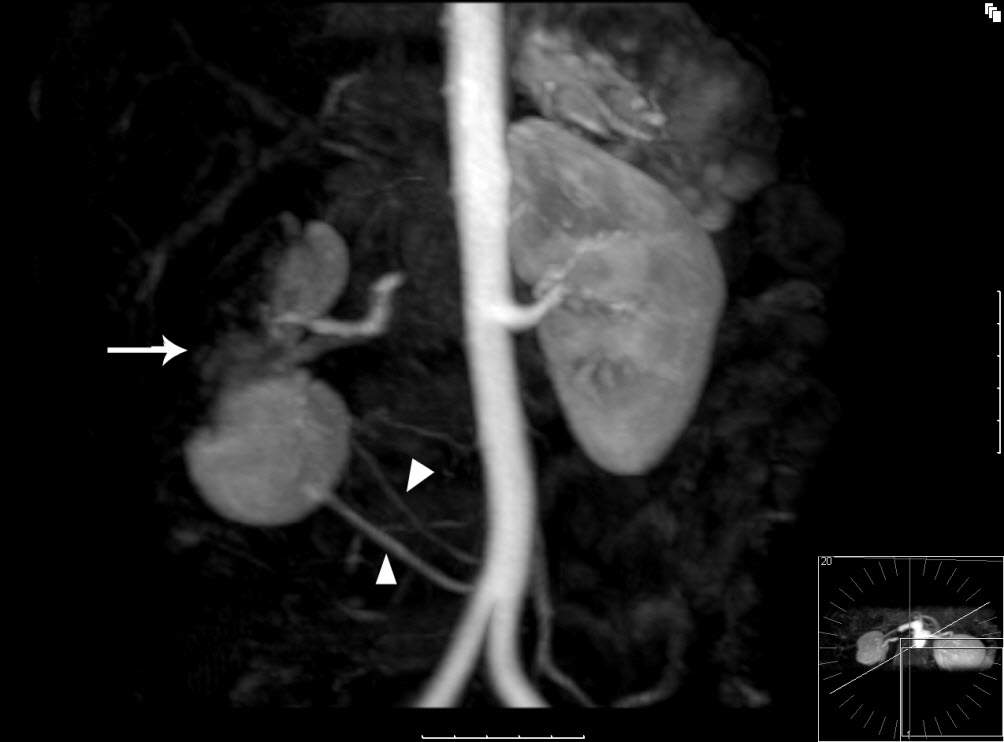 Figure 2 – MR angiographic 3D reconstruction: The lateral aspect and upper pole of the right kidney fails to enhance in keeping with renal infarction (white arrow). The inferior portion appears to enhance normally. This is due to a variant in the patient’s anatomy, with two small accessory renal arteries supplying the lower pole of the kidney (white triangles).
Figure 2 – MR angiographic 3D reconstruction: The lateral aspect and upper pole of the right kidney fails to enhance in keeping with renal infarction (white arrow). The inferior portion appears to enhance normally. This is due to a variant in the patient’s anatomy, with two small accessory renal arteries supplying the lower pole of the kidney (white triangles).
References
1. Stawicki SP, Rosenfeld JC, Weger N, Fields NL, Balshi JD Spontaneous renal artery dissection: three cases and clinical algorithms. Journal of Human Hypertension 2006; 20: 710–718
https://www.nature.com/jhh/journal/v20/n9/full/1002045a.html
2. Ramamoorthy SL, Vasquez JC, Taft PN, et al. Nonoperative management of acute spontaneous renal artery dissection. Annals Vasc Surg 2002;16(2): 157-16
https://www.sciencedirect.com/science/article/pii/S0890509607601447
3. Alamir A, Middendorf DF, Baker P, et al. Renal artery dissection causing renal infarction in otherwise healthy men. Am J Kidney Dis. 1997; 30: 851–855
https://www.sciencedirect.com/science/article/pii/S0272638697900949
4. Mudrick D, Arepally A, Geschwind JF, Ronsivalle JA, Lund GB, Scheel P. Spontaneous renal artery dissection: treatment with coil embolization. J Vasc Interv Radiol 2003; 14(4): 497-500
https://www.sciencedirect.com/science/article/pii/S105104430760236X
5. Müller BT, Reiher L, Pfeiffer T, Müller W, Hort W, Voiculescu A, Grabensee B, Fürst G, Sandmann W. Surgical treatment of renal artery dissection in 25 patients: indications and results. J Vasc Surg. 2003 37(4): 761-8
https://www.sciencedirect.com/science/article/pii/S0741521402753123
Date added to bjui.org: 20/11/2012
DOI: 10.1002/BJUIw-2012-083-web