Article of the Week: 3D percutaneous navigation, integrating position-tracking with a tablet display
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Dr. Osamu Ukimura, discussing his paper.
If you only have time to read one article this week, it should be this one.
Three-dimensional navigation system integrating position-tracking technology with a movable tablet display for percutaneous targeting
OBJECTIVES
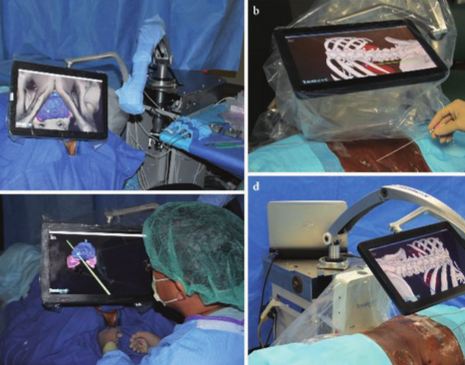
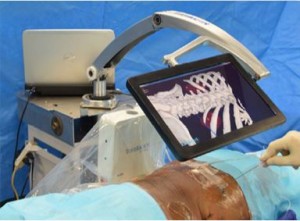
To assess the feasibility of a novel percutaneous navigation system (Translucent Medical, Inc., Santa Cruz, CA, USA) that integrates position-tracking technology with a movable tablet display.
MATERIALS AND METHODS
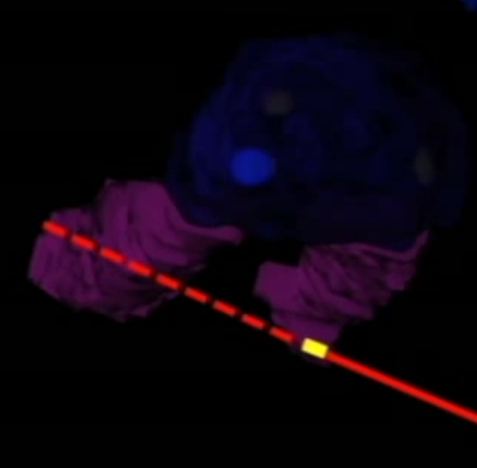
A total of 18 fiducial markers, which served as the target centres for the virtual tumours (target fiducials), were implanted in the prostate and kidney of a fresh cadaver, and preoperative computed tomography (CT) was performed to allow three-dimensional model reconstruction of the surgical regions, which were registered on the body intra-operatively. The position of the movable tablet’s display could be selected to obtain the best recognition of the interior anatomy. The system was used to navigate the puncture needle (with position-tracking sensor attached) using a colour-coded, predictive puncture-line. When the operator punctured the target fiducial, another fiducial, serving as the centre of the ablative treatment (treatment fiducial), was placed. Postoperative CT was performed to assess the digitized distance (representing the real distance) between the target and treatment fiducials to evaluate the accuracy of the procedure.
RESULTS
The movable tablet display, with position-tracking sensor attached, enabled the surgeon to visualize the three-dimensional anatomy of the internal organs with the help of an overlaid puncture line for the puncture needle, which also had a position-tracking sensor attached. The mean (virtual) distance from the needle tip to the target (calculated using the computer workstation), was 2.5 mm. In an analysis of each digitalized axial component, the errors were significantly greater along the z-axis (P < 0.01), suggesting that the errors were caused by organ shift or deformation.
CONCLUSION
This virtual navigation system, integrating a position-tracking sensor with a movable tablet display, is a promising advancement for facilitating percutaneous interventions. The movable display over the patient shows a preoperative three-dimensional image that is aligned to the patient. Moving the display moves the image, creating the feeling of looking through a window into the patient, resulting in instant perception and a direct, intuitive connection between the physician and the anatomy.