CRYPTORCHIDISM WITH BILATERAL MALIGNANT TRANSFORMATION
Introduction: The incidence of bilateral testicular germ-cell tumour (BGCT) ranges between 1 and 5% [1]. Synchronous bilateral germ cell tumour (BGCT) of the testis is rare and its association with bilateral cryptorchidism is even rarer.
Case report: 26year old male patient presented with lump in left iliac fossa since 3 months. USG guided FNAC of left iliac fossa lump and right sided inguinal testis was done which revealed seminoma bilaterally. Exploratory laparotomy done. Both lumps were excised. Histopathological examination of left side revealed classical seminoma and right side lump revealed intratubular germ cell neoplasm.
Discussion: Incidence of BGCT is much greater in patients in the younger age group as was in our case compared to that of unilateral seminoma which is common in patients older than 30 years. Synchronous testicular tumors commonly have concordant pathology in both testes as was seen in our case.
CONCLUSIONS: Importance of clinical examination and a simple imaging modality like USG could have possibly resulted in earlier diagnosis of the condition. Thus a thorough clinical and radiological evaluation is of paramount importance in patients.
Authors: Singh, Gurjit; Ali, Iqbal; Mahamia, Saurabh; Dnyanmote, Anuradha; Reddy,Raghuveer
Case Summary
A 26year old male patient presented with complaints of pain in the left iliac fossa and left lumbar region for 2 months. There was No history of vomiting, weight loss and altered bowel/bladder habits. Patient had been married for 10 years, but had no child and the couple was being investigated for infertility. Abdominal examination revealed a fixed, diffuse non tender lump in left iliac fossa measuring 9cms x 8cms which was firm in consistency and had a nodular surface. External genital examination revealed an empty scrotal sac. Routine investigations were normal but semen analysis revealed Azoospermia. Beta HCG level was 1.49 mIU/ml & Alpha Feto Protein level was 3.26 IU/ml which were normal, but LDH level was elevated to 378 IU/L.
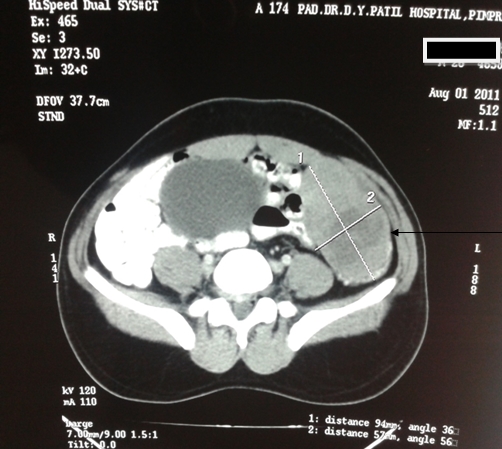
USG abdomen & pelvis revealed heterogeneous a mass in left iliac fossa with areas of cystic changes, and the right testis at the deep inguinal ring with specks of calcification. USG guided FNAC of left iliac fossa lump and right sided inguinal testis was done which revealed seminoma bilaterally (figure 4). Contrast Enhanced scan of Abdomen and Pelvis revealed a well defined lesion measuring 12cm x 9.8cm x 5.5cm in the left side of pelvis, with minimal displacement of bladder to the right. There was with minimal heterogenous enhancement with few areas of necrosis (figure 1). The fat planes of the lesion with posterior and medial bowel loops were blurred. There was minimal perilesional collection of free fluid in the left iliac fossa. Another well defined soft tissue density structure measuring 4cm x 3cm x 2cm with spermatic cord extending into the inguinal canal on the right side was suggestive of undescended testis. The scrotal sac was empty on either side with no evidence of pre- or para-aortic lymphadenopathy.
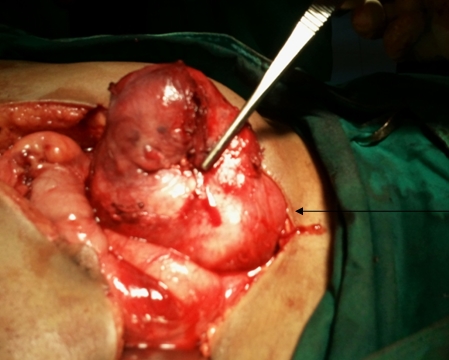
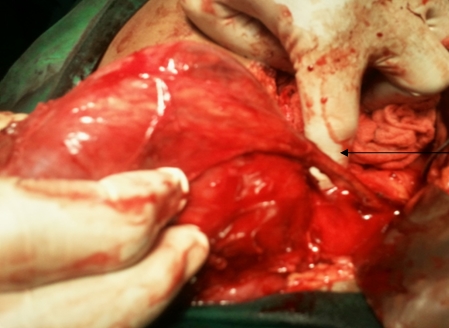
Both masses were approached through a transperitoneal paraumbilical midline incision. A 10cms x 6cms firm, smooth retroperitoneal mass in the left iliac region, which was adherent partially to parietal peritoneum and to a loop of small bowel, was excised in toto. It had a pedicle which was transfixed, ligated & divided near the deep inguinal ring (Figure 2). A soft mass measuring 4cm x 3cm was also excised in right iliac fossa after securing & dividing its pedicle (Figure 3).
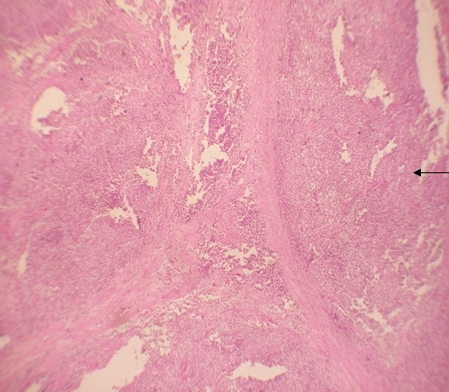
Histopathological examination of left side revealed classical seminoma (figure 5) and right side lump revealed intratubular germ cell neoplasm (figure 6 & 7). The postoperative period was uneventful. Diagnosis was identified as stage 1A bilateral synchronous seminoma with bilateral cryptoorchidism. Three cycles of adjuvant chemotherapy consisting of Inj. Bleomycin, Inj. Cisplatin & Inj. Etoposide I.V. were given at intervals of 21 days. Inj. Bleomycin I.V. in the dose of 31 mg, Inj. Cisplatin I.V. in the dose of 154 mg and Inj. Etoposide I.V. in the dose of 46.2 mg were administered. During the last three monthly intervals, the patient underwent clinical examination, and USG examination of Abdomen and estimation of Tumour markers was carried out.
Discussion
The incidence of bilateral germ cell tumour (BGCT) is much greater in patients in the younger age group, as in our case, compared to that of unilateral seminoma which is common in patients older than 30 years [1]. Testicular intratubular germ-cell neoplasia as seen on the right side in our case is considered a precursor lesion for developing germ-cell tumours [1]. In a study by Ch Theodore et al. of 45 patients, 31 had metachronous tumours and 14 patients had synchronous tumours. Nine patients had cryptorchidism that was bilateral in five. Our patient had synchronous tumours and had bilateral cryptorchidism. Bilateral germ cell tumours (BGCT) occur metachronously in 80-85% of cases and synchronously in 15-20% of cases [2]. Among patients presenting with seminoma, 1.8% develop BGCT as compared to 0.6% with nonseminomatous germ cell tumour (NSGCT) [2]. Synchronous testicular tumours commonly have concordant pathology in both testes as was seen in our case.
Several potential risk factors for developing a second testicular tumour are atrophy of the second testis, young age, infertility, and a family history of testicular cancer, atypical naevi, Down’s syndrome, and testicular maldescent.
Cryptorchidism is a known risk factor for the development of a testicular germ cell tumour [2]. Bilateral synchronous seminoma with bilateral cryptorchidism is rare. Our case falls in this rare category. In a study by Gezi L et al of 2386 patients, incidence of synchronous testicular tumour was 0.8% [3].
Incidence of intratubular germ cell neoplasia (IGCN) is high in patients with one or more risk factors (35-85%) and its detection allows curative tumour eradication with minimal morbidity or mortality, along with the possibility of preserving testicular function. In our case IGCN was associated with cryptorchidism, hence it needed orchidectomy. In a study by Coogan cl et al. of 2088 patients with testicular carcinoma at Indiana University, 21 patients (1%) were identified with bilateral testicular carcinoma. Amongst these, sixteen patients had metachronous tumours, and only five patients were with synchronous testicular tumours [4]. In a study by Che M et al. of 2431 patients with testicular germ cell tumours, only 24 patients were identified having bilateral germ cell tumours. Overall incidence of bilateral germ cell tumours in the patients with testicular germ cell tumours was 1%. Primary bilateral testicular tumour is rare; its prevalence ranges from 2.5% to 5%, most tumours being metachronous, however our case falls in the rare category of synchronous tumours. In a study by Tekin et al. of 552 patients with GCTT, 11 patients developed bilateral disease. Out of 11 patients, 7 developed a second tumour metachronously and four had synchronous bilateral GCTT. The incidence of bilateral germ cell tumours was 1.8% (14 of 776 patients) in patients with seminoma and 0.6% (10 of 1655 patients) in patients with nonseminomatous germ cell tumours [5]. Cryptoorchidism, infertility or atrophic testis was associated with development of bilateral GCTT in seven of the 11 patients. All synchronous tumours and most of the sequential tumours had identical histology on both sides. Intramuscular testosterone was administered periodically after total castration [7]. In our case we have given Testosterone cypionate 200 mg IM every 2 weeks for 6 months.
There is a lot of heterogeneity in the reported series regarding the management of synchronous BGCT and only broad generalizations can be made from these. Post-orchidectomy management of these patients has been dictated by the stage of the tumour in either of the testes and the pathology with the higher malignant potential (NSGCT as compared to pure seminoma) [3]. Bilateral seminomas have a higher tumour burden, therefore these patients should not be kept on surveillance only; rather they should be treated with prophylactic para-aortic lymph node irradiation or one to two cycles of adjuvant chemotherapy. Patients in Stage II or higher should be treated with chemotherapy [2]. In our case, the patient having been placed in stage 1A, chemotherapy was chosen as the modality of adjuvant treatment.
For selected patients with tumours smaller than 25 mm confined to the testis and with normal preoperative testosterone, testis sparing surgery (TSS) to avoid lifelong androgen replacement and preservation of fertility should be offered to patients who are aware and accept the risk of a subsequent local relapse and who realize the importance of compliance during follow-up. Patients not suitable for TSS should be offered testosterone replacement therapy after bilateral orchidectomy, as has been done in our case.
Conclusions
Clinical examination and a simple imaging modality like USG could possibly have resulted in earlier diagnosis of the condition. Thus a thorough clinical and radiological evaluation is of paramount importance in patients being investigated for infertility. Bilateral testicular tumours with cryptorchidism are extremely rare and amongst them synchronous bilateral testicular tumours are even rarer. Adjuvant therapy in the form of cisplatin-based chemotherapy has markedly increased the survival rate, which should augur well for this patient. Prognosis depends upon the clinical staging and not whether it is unilateral or bilateral involvement, hence is favourable in this case. Our patient will require lifelong follow up and androgen replacement therapy.
References
1. Ch Theodore, M J Terrier-Lacombe, A Laplanche, G Benoit, K Fizazi, O Stamerra and P Wibault. Bilateral germ-cell tumours: 22-year experience at the Institute Gustavo Roussy. Br J Cancer. 2004; 90(1):55–59.
2. Sushma Agrawal, Ranjeet Bajpai, R. K. Agrawal and T. C. Gupta. Bilateral synchronous seminoma with bilateral cryptorchidism of the testis. Indian J Urol.2010; 26(4):587–589
3. Walid K. Adham, Bharat K. Raval, Maria C. Uzquiano and Luciano B. Lemos., Bilateral Testicular Tumors: Seminoma and Mixed Germ Cell Tumor. Jrnl of RadioGraphics. 2005; 25:835-839.
4. Coogan CL, Foster RS, Simmons GR, Tognoni PG, Roth BJ, Donohue JP. Bilateral testicular tumors: management and outcome in 21 patients. Cancer. 1998; 83(3):547-52.
5. Che M, Tamboli P, Ro JY, Park DS, Ro JS, Amato RJ, Ayala AG. Bilateral testicular germ cell tumors: Twenty-year experience at M. D. Anderson Cancer Center. Br J Cancer. 2002; 95(6):1228-33.
6. García Morúa A, Gutiérrez García JD, Ortiz Lara Gerardo E, Martínez Montelongo R, Gómez Guerra Lauro S. Synchronous bilateral testicular seminoma in an adult patient with bilateral cryptorchidism: A case report and literature review.Actas Urol Esp.2010;34(2):210-1.
7. Tekin A, Aygun YC, Aki FT, Ozen H. Bilateral germ cell cancer of the testis: A report of 11 patients with a long-term follow-up. BJU Int. 2000; 85(7):864-8.
8. Géczi L, Gomez F, Bak M, Bodrogi I. The incidence, prognosis, clinical and histological characteristics, treatment, and outcome of patients with bilateral germ cell testicular cancer in Hungary. J Cancer Res Clin Oncol. 2003; 129(5):309-15.
9. HolzbeierleinJM, Sogani PC, Sheinfeld J. Histology and clinical outcomes in patients with bilateral testicular germ cell tumors: the Memorial Sloan Kettering Cancer Center experience 1950 to 2001. J Urol.2003; 169(6):2122-5.
Figure 1: Ct scan showing retroperitoneal mass in left iliac fossa
Figure 2: Retroperitoneal mass in left iliac fossa
Figure 3: Mass in right iliac fossa with pedicle
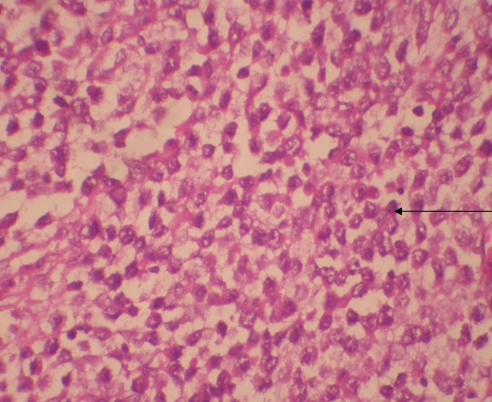
Figure 4: Tumour cells with sheets separated by fibrous septa (10x view)
Figure 5: Seminoma cells showing moderate amount of cytoplasm and hyperchromatic cells (40 X View)
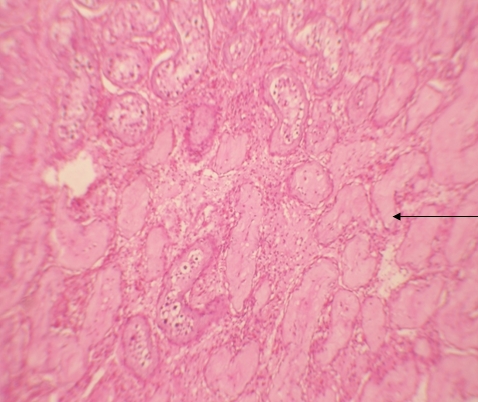
Figure 6: Section shows seminiferous tubules (ITGN) (10 x view)
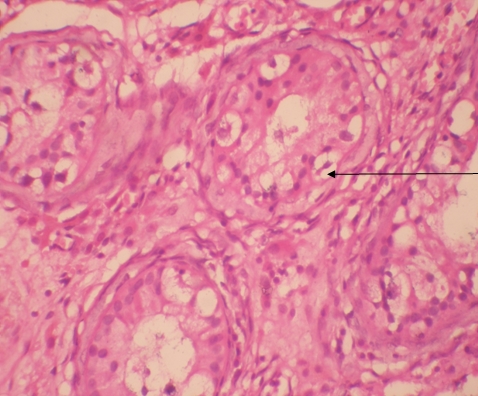
Figure 7: Leydig cells in ITGN with clear cytoplasm basally placed (40 x view)
Date added to bjui.org: 14/11/2012
DOI: 10.1002/BJUIw-2012-061-web