Article of the Week: URB937 reduces PGE2-induced bladder overactivity
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
URB937, a peripherally restricted inhibitor for fatty acid amide hydrolase, reduces prostaglandin E2-induced bladder overactivity and hyperactivity of bladder mechano-afferent nerve fibres in rats
Objective
To determine if inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) can counteract the changes in urodynamic variables and bladder afferent activities induced by intravesical prostaglandin E2 (PGE2) instillation in rats.
Materials and methods
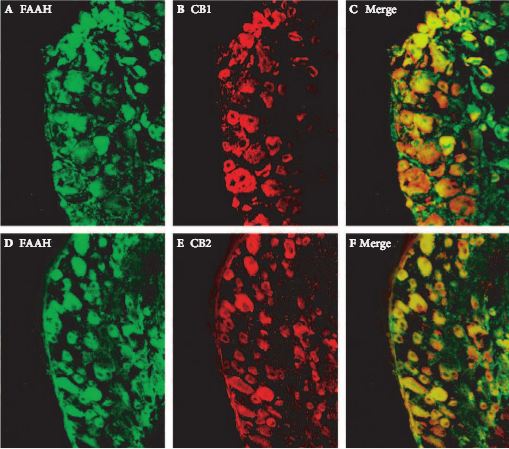
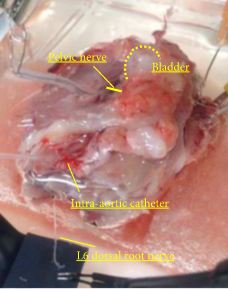
In female Sprague–Dawley rats we studied the effects of URB937, a peripherally restricted FAAH inhibitor, on single-unit afferent activity (SAA) during PGE2-induced bladder overactivity (BO). SAA measurements were made in urethane-anaesthetised rats and Aδ- and C-fibres were identified by electrical stimulation of the pelvic nerve and by bladder distention. Cystometry (CMG) in conscious animals and during SAA measurements was performed during intravesical instillation of PGE2 (50 or 100 μm) after intravenous administration of URB937 (0.1 and 1 mg/kg) or vehicle. In separate experiments, the comparative expressions of FAAH and cannabinoid receptors, CB1 and CB2, in microsurgically removed L6 dorsal root ganglion (DRG) were studied by immunofluorescence.
Results
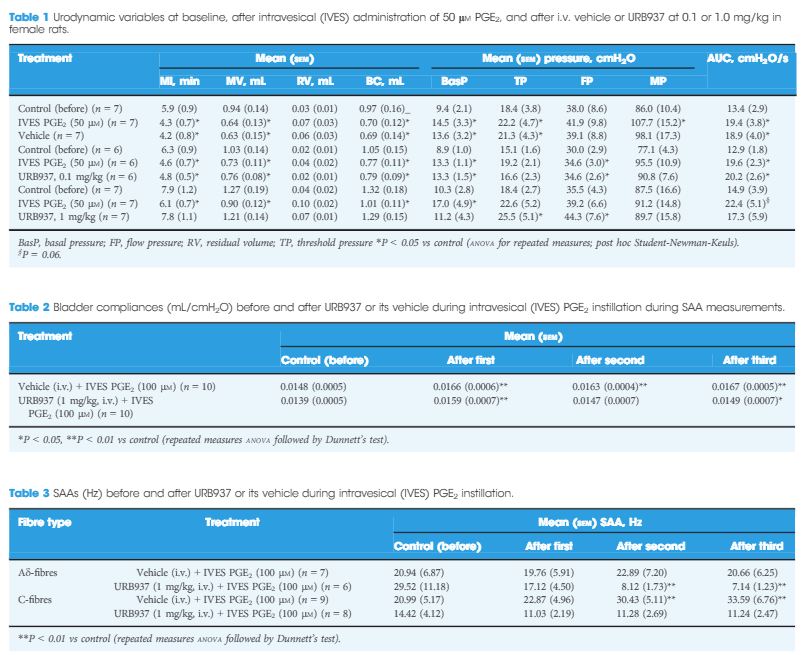
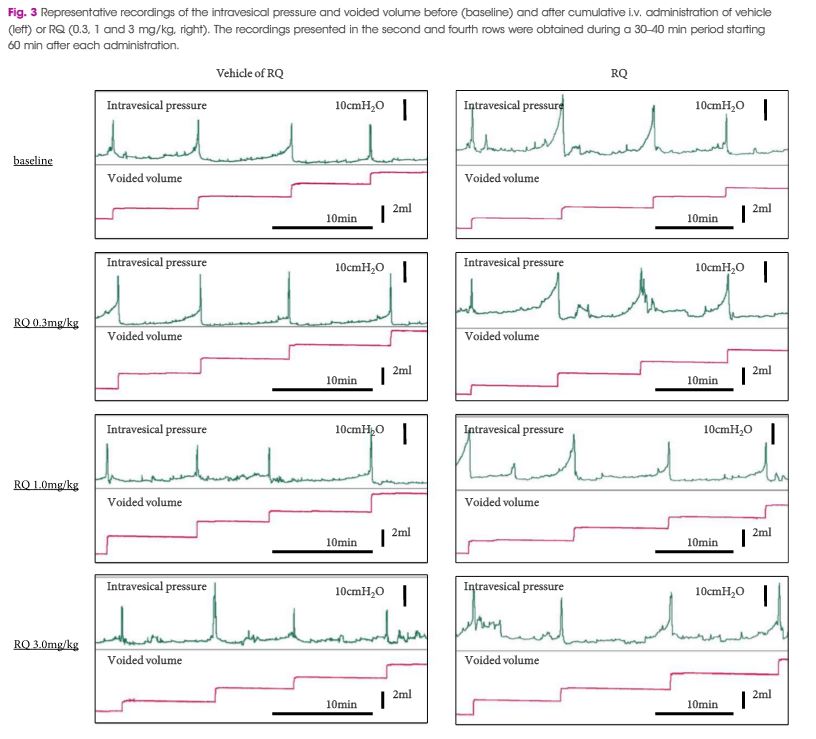
During CMG, 1 mg/kg URB937, but not vehicle or 0.1 mg/kg URB937, counteracted the PGE2-induced changes in urodynamic variables. PGE2 increased the SAAs of C-fibres, but not Aδ-fibres. URB937 (1 mg/kg) depressed Aδ-fibre SAA and abolished the facilitated C-fibre SAA induced by PGE2. The DRG nerve cells showed strong staining for FAAH, CB1 and CB2, with a mean (sem) of 77 (2)% and 87 (3)% of FAAH-positive nerve cell bodies co-expressing CB1 or CB2 immunofluorescence, respectively.
Conclusion
The present results show that URB937, a peripherally restricted FAAH inhibitor, reduces BO and C-fibre hyperactivity in the rat bladder provoked by PGE2, suggesting an important role of the peripheral endocannabinoid system in BO and hypersensitivity.