Article of the week: Use of 68Ga-PSMA/PET for detecting lymph node metastases in primary and recurrent PCa and location of recurrence after radical prostatectomy: an overview of the current literature
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
If you only have time to read one article this week, we recommend this one.
Use of gallium‐68 prostate‐specific membrane antigen positron‐emission tomography for detecting lymph node metastases in primary and recurrent prostate cancer and location of recurrence after radical prostatectomy: an overview of the current literature
Henk B. Luiting*, Pim J. van Leeuwen†, Martijn B. Busstra*, Tessa Brabander‡, Henk G. van der Poel†, Maarten L. Donswijk§, André N. Vis¶, Louise Emmett**††, Phillip D. Stricker‡‡§§¶¶ and Monique J. Roobol*
*Department of Urology, Erasmus University Medical Centre, Rotterdam, †Department of Urology, Netherlands Cancer Institute, Amsterdam, ‡Department of Radiology and Nuclear Medicine, Erasmus University Medical Centre, Rotterdam, §Department of Nuclear Medicine, Netherlands Cancer Institute, ¶Department of Urology, Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands, **Department of Nuclear Medicine, St Vincent’s Hospital, ††University of New South Wales, Sydney, ‡‡St. Vincent’s Prostate Cancer Centre, §§Garvan Institute of Medical Research, Kinghorn Cancer Centre, Darlinghurst and ¶¶St Vincent’s Clinical School, UNSW, Sydney, NSW, Australia
Abstract
Objectives
To review the literature to determine the sensitivity and specificity of gallium‐68 prostate‐specific membrane antigen (68Ga‐PSMA) positron‐emission tomography (PET) for detecting pelvic lymph node metastases in patients with primary prostate cancer (PCa), and the positive predictive value in patients with biochemical recurrence (BCR) after initial curative treatment, and, in addition, to determine the detection rate and management impact of 68Ga‐PSMA PET in patients with BCR after radical prostatectomy (RP).
Materials and Methods
We performed a comprehensive literature search. Search terms used in MEDLINE, EMBASE and Science Direct were ‘(PSMA, 68Ga‐PSMA, 68Gallium‐PSMA, Ga‐68‐PSMA or prostate‐specific membrane antigen)’ and ‘(histology, lymph node, staging, sensitivity, specificity, positive predictive value, recurrence, recurrent or detection)’. Relevant abstracts were reviewed and full‐text articles obtained where possible. References to and from obtained articles were searched to identify further relevant articles.
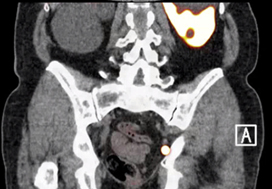
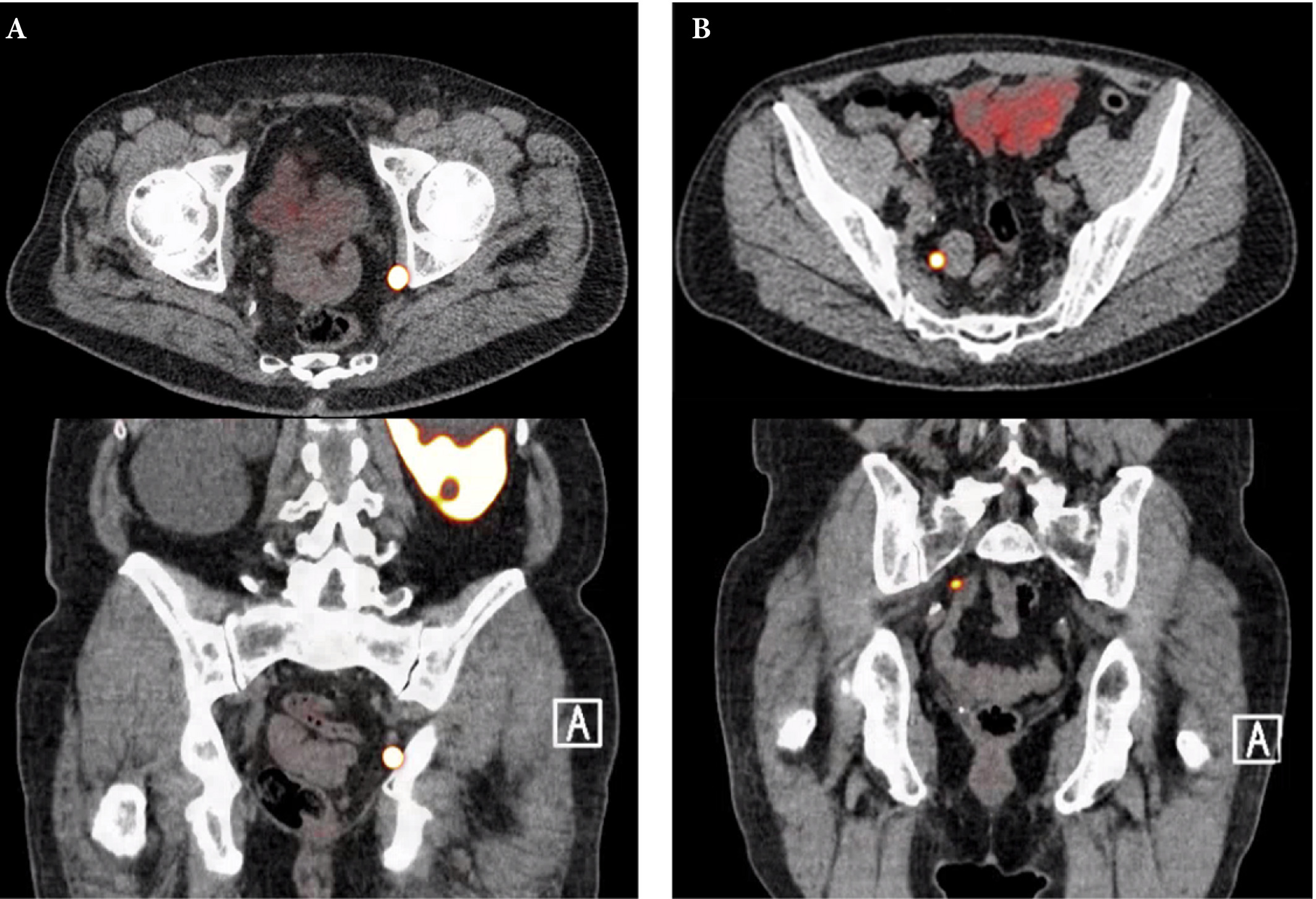 Fig. 1. Axial and sagittal plane gallium‐68 prostate‐specific membrane antigen positron‐emission tomography /CT images of two patients with locoregional lymph node recurrence after initial curative treatment. The metastasis in patient A is located in the obturator area and the metastasis in patient B is located in the presacral area.
Fig. 1. Axial and sagittal plane gallium‐68 prostate‐specific membrane antigen positron‐emission tomography /CT images of two patients with locoregional lymph node recurrence after initial curative treatment. The metastasis in patient A is located in the obturator area and the metastasis in patient B is located in the presacral area.
Results
Nine retrospective and two prospective studies described the sensitivity and specificity of 68Ga‐PSMA PET for detecting pelvic lymph node metastases before initial treatment, which ranged from 33.3% to 100% and 80% to 100%, respectively. In eight retrospective studies, the positive predictive value of 68Ga‐PSMA PET in patients with BCR before salvage lymph node dissection ranged from 70% to 100%. The detection rate of 68Ga‐PSMA PET in patients with BCR after RP in the PSA subgroups <0.2 ng/mL, 0.2–0.49 ng/mL and 0.5 to <1.0 ng/mL ranged from 11.3% to 50.0%, 20.0% to 72.7% and 25.0% to 87.5%, respectively.
Conclusion
The review results showed that 68Ga‐PSMA PET had a high specificity for the detection of pelvic lymph node metastases in primary PCa. Furthermore, 68Ga‐PSMA PET had a very high positive predictive value in detecting lymph node metastases in patients with BCR. By contrast, sensitivity was only moderate; therefore, based on the currently available literature, 68Ga‐PSMA PET cannot yet replace pelvic lymph node dissection to exclude lymph node metastases. In the salvage phase, 68Ga‐PSMA PET had both a high detection rate and impact on radiotherapy planning in early BCR after RP.