Article of the Month: Indications for Intervention During Active Surveillance of Prostate Cancer
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Dr. Max Kates discussing his paper.
If you only have time to read one article this week, it should be this one.
Indications for Intervention During Active Surveillance of Prostate Cancer: A Comparison of the Johns Hopkins and PRIAS Protocols
OBJECTIVE
To analyse how patients enrolled in our biopsy based surveillance programme would fare under the Prostate Cancer Research International Active Surveillance (PRIAS) protocol, which uses PSA kinetics.
PATIENTS AND METHODS
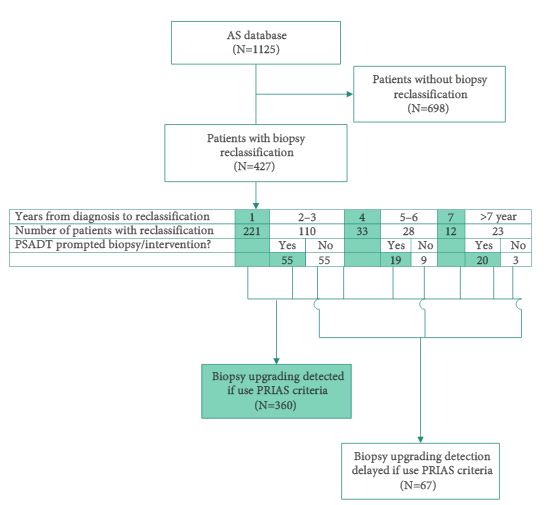
Since 1995, 1125 men with very-low-risk prostate cancer have enrolled in the AS programme at the Johns Hopkins Hospital (JHH), which is based on monitoring with annual biopsy. The PRIAS protocol uses a combination of periodic biopsies (in years 1, 4, and 7) and prostate-specific antigen doubling time (PSADT) to trigger intervention. Patients enrolled in the JHH AS programme were retrospectively reviewed to evaluate how the use of the PRIAS protocol would alter the timing and use of curative intervention.
RESULTS
Over a median of 2.1 years of follow up, 38% of men in the JHH AS programme had biopsy reclassification. Of those, 62% were detected at biopsy intervals corresponding to the PRIAS criteria, while 16% were detected between scheduled PRIAS biopsies, resulting in a median delay in detection of 1.9 years. Of the 202 men with >5 years of follow-up, 11% in the JHH programme were found to have biopsy reclassification after it would have been identified in the PRIAS protocol, resulting in a median delay of 4.7 years to reclassification. In all, 12% of patients who would have undergone immediate intervention under PRIAS due to abnormal PSA kinetics would never have undergone reclassification on the JHH protocol and thus would not have undergone definitive intervention.
CONCLUSIONS
There are clear differences between PSA kinetics-based AS programmes and biopsy based programmes. Further studies should address whether and how the differences in timing of intervention impact subsequent disease progression and prostate cancer mortality.