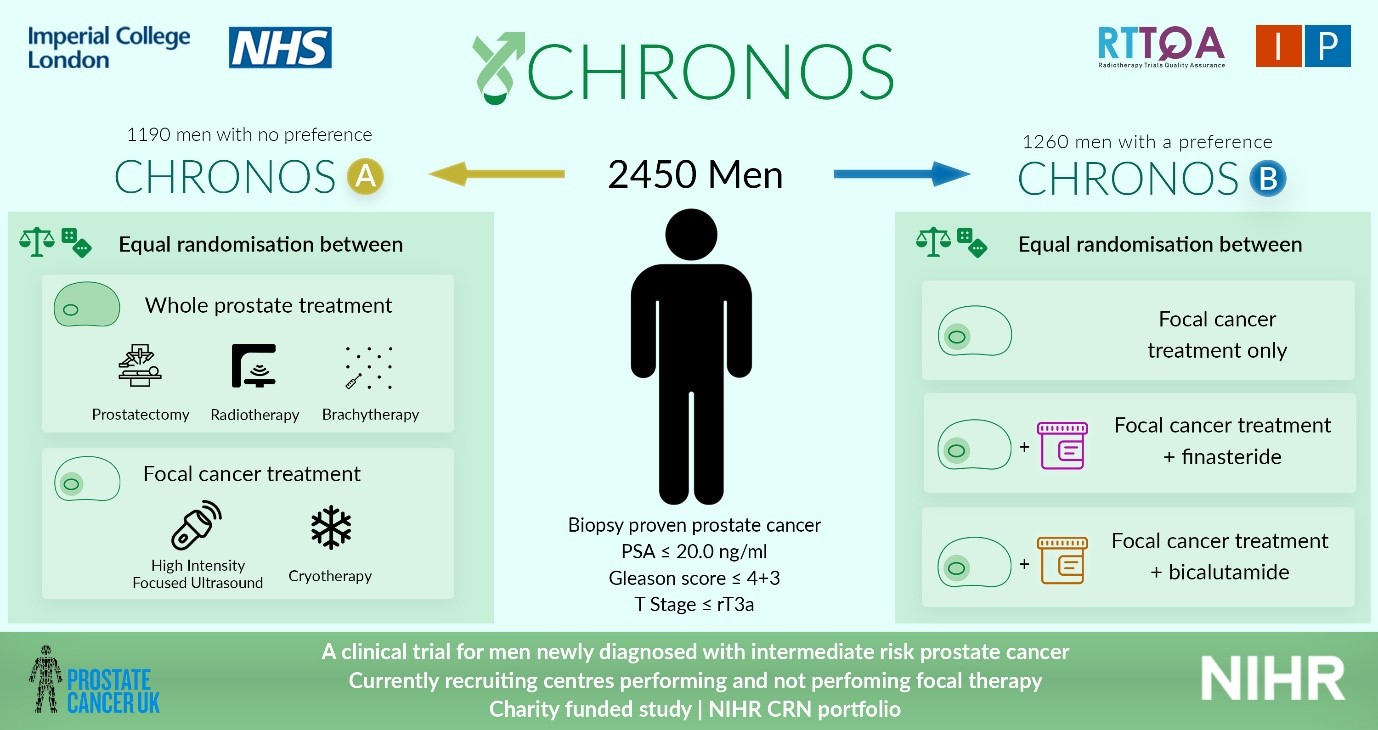
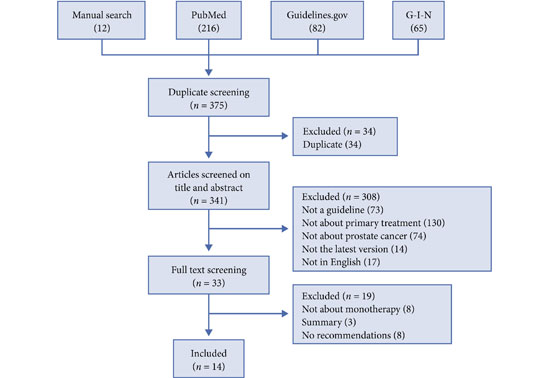
Sir,
Thank you for your interest in our article regarding whole-gland brachytherapy to the prostate for prostate cancer (1). Your letter is highlighting the expanding role of brachytherapy to that of focal therapy (2). We agree that multiparametric magnetic resonance imaging (mpMRI) scans have expanded the ability to localise tumours and indeed that they may be useful in carefully selected men wishing to undergo focal therapy. However, other advances such as the use of fiducial markers and spacers have also allowed better dosimetry and for a reduction in side effects (3). The safety and performance of brachytherapy in whole gland treatment means we should have faith in it as a modality to destroy cancer on the focal therapy setting. There are new trials being developed with focal brachytherapy and we look forward to the results in the coming years.
References
- Chao MW, Grimm P, Yaxley J, Jagavkar R, Ng M, Lawrentschuk N. Brachytherapy: state-of-the-art radiotherapy in prostate cancer. BJU Int 2015; 116(S3): 80-8. doi: 10.1111/bju.13252.
- Nguyen PL, Trachtenberg J, Polascik TJ. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol. 2014 Oct;66(4):732-51. doi: 10.1016/j.eururo.2013.05.048. Epub 2013 Jun 6. Review.
- Ng M1, Brown E, Williams A, Chao M, Lawrentschuk N, Chee R. BJU Int. 2014 Mar;113 Suppl 2:13-20. doi: 10.1111/bju.12624. Fiducial markers and spacers in prostate radiotherapy: current applications.
Letter to the Editor
Opportunity of widening the resort to multiparametric MRI/transrectal ultrasound fusion imaging-guided prostate cancer brachytherapy
Sir,
I have recently read, with high interest, the review article “Brachytherapy: state-of-the-art radiotherapy in prostate cancer”, by Chao et al.[1]. The authors made extremely clear the advanced technologies of computerized treatment planning and imaging-guided delivery modalities to reach a tailored ablative prostate tumor target dose by resorting to either low-dose-rate (LDR) or high-dose-rate (HDR) different brachytherapy procedures as regards three basic – low, intermediate, high – disease risk classificative conditions.
It is today proven that focal instrumental procedures inside the prostate gland – from biopsy to various prostate cancer focused ablative strategies, among which laser interstitial thermal therapy and particularly the prostate cancer brachytherapy – might require the resort to proper software digital overlay-mediated fusion of both beforehand multiparametric magnetic resonance imaging (mpMRI) scans and later real-time transrectal 3D ultrasound findings. Like this, indeed, intriguing developments in software modelling techniques have led to reach, by a mpMRI-ultrasound image fusion approach, more accurate targeted prostate cancer biopsies than those by transrectal ultrasound imaging alone achieved [2,3].
If the transperineal focal laser prostate tumor ablation usually occurs only with the guidance of mpMRI (T2-weighted, diffusion-weighted, dynamic contrast material) [4], as regards the prostate cancer brachytherapy, instead, it is more and more timely, for just targeting the tumor “index-dominant lesion”, the resort to mpMRI/transrectal real-time ultrasound fusion imaging. Quite recently, mpMRI/real-time transrectal ultrasound software-mediated digital co-registration has allowed to properly carry-out, in patients suffering from intermediate/high risk prostate carcinoma with mpMRI visible “index- dominant” intraprostatic nodule, the HDR ¹⁹² Ir transperineal temporary implant-brachytherapy as accurate partial prostate radiation dose escalation supplemental to hypofractionated external beam radiotherapy [5,6].
Given the interesting, even rare, reports on this subject, it would be advisable to widen the resort to the above-outlined mpMRI/transrectal ultrasound fusion imaging-guided prostate cancer brachytherapy, particularly for a suitably targeted dominant tumor nodule detection/ablation.
Contardo Alberti
L D of Surgical Semeiotics, University of Parma, Parma, Italy
References
1 Chao MW, Grimm P, Yaxley J, Jagavkar R, Ng M, Lawrentschuk N. Brachytherapy: state-of-the-art radiotherapy in prostate cancer. BJU Int 2015; 116(S3): 80-8. doi: 10.1111/bju.13252.
2 Shoji S, Hiraiwa S, Endo J, Hashida K, Tomonaga T, Nakano M et al. Manually controlled targeted prostate biopsy with real-time fusion imaging of multiparametric magnetic resonance imaging and stransrectal ulrasound :an early experience. Int J Urol 2015; 22(2): 173-8. doi: 10.1111/iju.12643.
3 Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol 2013; 23(1): 43-50. doi: 10.1097/MOU.0b013e32835ad3ee.
4 Woodrum DA, Kawashima A, Gorny KR, Mynderse LA. Magnetic resonance-guided thermal therapy for localized and recurrent prostate cancer. Magn Reson Imaging Clin N Am.2015; 23(4): 607-19. doi:10.1016/j.mric.2015.05.014
5 Bubley GJ, Bloch BN, Vazquez C, Genega E, Holupka E, Rofsky N, Kaplan I. Accuracy of endorectal magnetic resonance/transrectal ultrasound fusion for detection of prostate cancer during brachytherapy. Urology 2013;81(6): 1284-9. doi: 10.1016/j.urology.2012.12.051.
6 Gomez-Iturriaga A, Casquero F, Urresola A, Ezquerro A, Lopez JI, Espinosa JM et al. Dose escalation to dominant intraprostatic lesions with MRI-transrectal ultrasound fusion high-dode-rate prostate brachytherapy. Radiother Oncol 2016 Feb 15. doi: 10.1016/j.radonc.2016.02.004 (Epub ahead of print).