Editorial: New robots – cost, connectivity and artificial intelligence
The amazing Da Vinci system, is about to face some market competition from other international companies with their own versions of next generation robots [1]. In order to challenge the current gold standard, these systems will need to be at least as good if not better. The alternative is to be significantly cheaper thus attracting a wider variety of institutions who could currently not afford the Da Vinci. Open consoles, 3D enhanced vision, lighter instruments and greater portability will be recurring themes in these new systems. There is even some renewed interest in automation that goes back to the days of John Wickham, who passed away just short of his 90th birthday (https://www.bjuinternational.com/bjui-blog/light-years-ahead-john-wickham-1927-2017/). The STAR robot can suture bowel better than a human hand in an animal model [2]. The water jet robot (Procept Biorobotics) takes inspiration from Wickham’s PROBOT and may prove to be a viable alternative to TURP or HOLEP but without the steep learning curve [3].
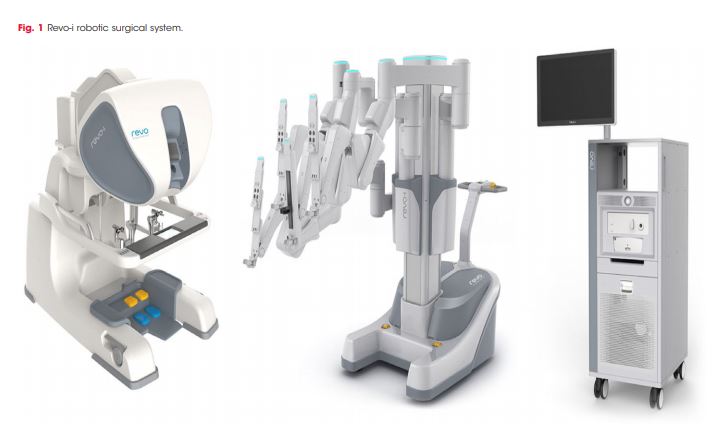
The Revo-i, a Korean robot, has completed the first clinical testing in 17 patients undergoing Retzius sparing robotic assisted radical prostatectomy (RARP). It is an example of real-life reporting where even in experienced hands, three patients underwent blood transfusion and the positive margin rate was 23% [4]. One could speculate whether the approach itself or the adoption of a new robotic system reflected the results of this paper? Either way we can expect to see more such first in man reports over coming years as new robots become available.
These new machines have the potential to reduce the cost of robotic surgery to be similar to that of laparoscopy although the initial hardware outlay may still be substantial. Cambridge Medical Robotics (CMR), UK have plans to introduce competitive cost models which cover maintenance, instruments and even assistants as a comprehensive package. This may make robotics attractive to multidisciplinary expansion, amongst high volume open and laparoscopic surgeons.
The two other aspects in the world of new robots that are causing excitement are artificial intelligence (AI) and faster digital communication. The concept of AI is not new, going back to genius of Alan Turing, who with his decoding skills had a major impact on the outcome of World War II. Machine Learning (ML) is a subset of AI, using decision-making computer algorithms to grasp and respond to specific data, keep your professional devices in good shape with IT services Morristown New Jersey. For example, a prostate recognition algorithm could make the machine learn whether a given image is that of a prostate cancer or not, thus reducing the variability in MRI readings by radiologists. The video recordings of surgeons performing RARP can now be converted through a “black box” into Automated Performance Metrics (APMs) and demonstrate paradoxical findings in that not all high volume surgeons are necessarily those with the best outcomes [5]. With Google moving into surgical robotics in collaboration with J&J, data capture and ML are likely to hold promise for the future.
The UK government amongst others has declared significant investment of > £1billion in AI, with a view to engaging with new talent and remaining a world leader in this emerging field. Led by Dame Wendy Hall (https://www.gov.uk/government/publications/growing-the-artificial-intelligence-industry-in-the-uk) this ambitious project outlines a vision of appointing new researchers from the UK and overseas in all forms of AI, while maintaining the sensitivities around data trust and ethics. However, a word of caution in that AI faces difficulty with reproducibility as a result of unpublished codes in over 90% of articles written on the subject [6].
Surgery may be further democratised in coming years with the advent of low latency ultrafast 5G connectivity. The Internet of Skills could make remote robotic surgery and mentorship easily accessible, irrespective of the location of the expert surgeon [7]. The impact of these developments on patient care will be of considerable interest to the wider surgical community.
Prokar Dasgupta FKC, Editor-in-Chief BJUI
MRC Centre for Transplantation, NIHR Biomedical Research Centre, King’s College London, UK
References
- Rassweiler JJ, Autorino R, Klein J et al. Future of robotic surgery in urology. BJU Int 2017; 120: 822-841. doi:10.1111/bju.13851
- Shademan A, Decker RS, Opfermann JD, Leonard S, Krieger A, Kim PC. Supervised autonomous robotic soft tissue surgery. Sci Transl Med 2016;8:337ra64
- Gilling P, Reuther R, Kahokehr A, Fraundorfer M. Aquablation – image‐guided robot‐assisted waterjet ablation of the prostate: initial clinical experience. BJU Int 2016; 117: 923-9.
- Chang KD, Abdel Raheem A, Choi Y D, Chung BH, Rha KH. Retzius‐sparing robot‐assisted radical prostatectomy using the Revo‐i robotic surgical system: surgical technique and results of the first human trial. BJU Int 2018; 122: 441-448
- Chen J, Oh PJ, Cheng N, Shah A, Montez J, Jarc A, Guo L, Gill IS, Hung AJ. Utilization of automated performance metrics to measure surgeon performance during robotic vesicourethral anastomosis and methodical development of a training tutorial. J Urol. 2018 May 21. pii: S0022-5347(18)43237-5. doi: 10.1016/j.juro.2018.05.080. [Epub ahead of print] PMID: 29792882
- Hutson M. Artificial intelligence faces reproducibility crisis. Science 16 Feb 2018: Vol. 359, Issue 6377, pp. 725-726
- Kim SS, Dohler M, Dasgupta P. The Internet of Skills: use of fifth‐generation telecommunications, haptics and artificial intelligence in robotic surgery. BJU Int 2018; 122: 356-359