Article of the Month: MRI supported transperineal prostate biopsy
Every Month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy‐naïve men with suspicion of prostate cancer
Abstract
Objectives
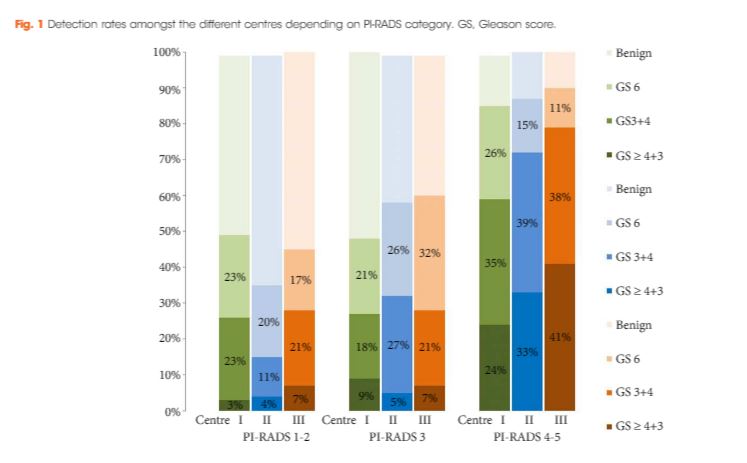
To analyse the detection rates of primary magnetic resonance imaging (MRI)‐fusion transperineal prostate biopsy using combined targeted and systematic core distribution in three tertiary referral centres.
Patients and Methods
In this multicentre, prospective outcome study, 807 consecutive biopsy‐naïve patients underwent MRI‐guided transperineal prostate biopsy, as the first diagnostic intervention, between 10/2012 and 05/2016. MRI was reported following the Prostate Imaging‐Reporting and Data System (PI‐RADS) criteria. In all, 236 patients had 18–24 systematic transperineal biopsies only, and 571 patients underwent additional targeted biopsies either by MRI‐fusion or cognitive targeting if PI‐RADS ≥3 lesions were present. Detection rates for any and Gleason score 7–10 cancer in targeted and overall biopsy were calculated and predictive values were calculated for different PI‐RADS and PSA density (PSAD) groups.
Results
Cancer was detected in 68% of the patients (546/807) and Gleason score 7–10 cancer in 49% (392/807). The negative predictive value of 236 PI‐RADS 1–2 MRI in combination with PSAD of <0.1 ng/mL/mL for Gleason score 7–10 was 0.91 (95% confidence interval ± 0.07, 8% of study population). In 418 patients with PI‐RADS 4–5 lesions using targeted plus systematic biopsies, the cancer detection rate of Gleason score 7–10 was significantly higher at 71% vs 59% and 61% with either approach alone (P < 0.001). For 153 PI‐RADS 3 lesions, the detection rate was 31% with no significant difference to systematic biopsies with 27% (P > 0.05). Limitations include variability of multiparametric MRI (mpMRI) reading and Gleason grading.
Conclusion
MRI‐based transperineal biopsy performed at high‐volume tertiary care centres with a significant experience of prostate mpMRI and image‐guided targeted biopsies yielded high detection rates of Gleason score 7–10 cancer. Prostate biopsies may not be needed for men with low PSAD and an unsuspicious MRI. In patients with high probability lesions, combined targeted and systematic biopsies are recommended.