Article of the Week: LCA – EuRECA study
Every week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Oncological outcomes and complication rates after laparoscopic-assisted cryoablation: a European Registry for Renal Cryoablation (EuRECA) multi-institutional study
Abstract
Objective
To assess complication rates and intermediate oncological outcomes of laparoscopic-assisted cryoablation (LCA) in patients with small renal masses (SRMs).
Patients and Methods
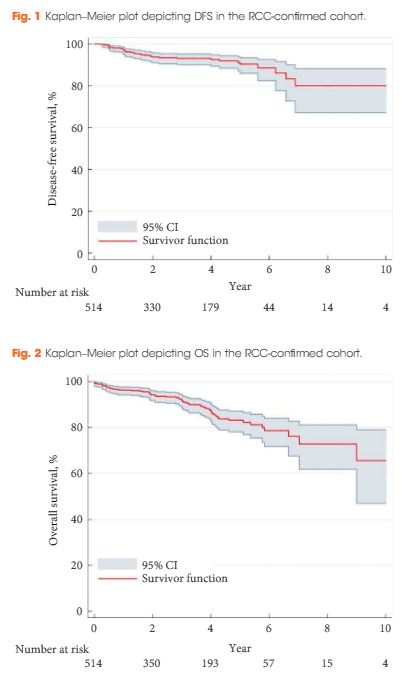
A retrospective review of 808 patients treated with LCA for T1a SRMs from 2005 to 2015 at eight European institutions. Complications were analysed according to the Clavien–Dindo classification. Kaplan–Meier analyses were used to estimate 5- and 10-year disease-free survival (DFS) and overall survival (OS).
Results
The median [interquartile (IQR)] age was 67 (58–74) years. The median (IQR) tumour size was 25 (19–30) mm. The transperitoneal approach was used in 77.7% of the patients. The median postoperative hospital stay was 2 days. In all, 514 patients with a biopsy-confirmed renal cell carcinoma (RCC) were available for survival analyses. The median (IQR) follow-up for the RCC-cohort was 36 (14–56) months. A total of 32 patients (6.2%) were diagnosed with treatment failure. The 5-/10-year DFS was 90.4%/80.0% and 5-/10-year OS was 83.2%/64.4%, respectively. A total of 134 postoperative complications (16.6%) were reported, with severe complications (grade ≥III) in 26 patients (3.2%). An American Society of Anesthesiologists score of 3 was associated with an increased risk of overall complications (odds ratio 2.85, 95% confidence interval 1.32–6.20; P = 0.005).
Conclusions
This large series of LCA demonstrates satisfactory long-term oncological outcomes for SRMs. However, although LCA is considered a minimally invasive procedure, risk of complications should be considered when counselling patients.