Article of the Week: ERSPC risk calculators significantly outperform the PCPT 2.0 in the prediction of PCa
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
European Randomised Study of Screening for Prostate Cancer (ERSPC) risk calculators significantly outperform the Prostate Cancer Prevention Trial (PCPT) 2.0 in the prediction of prostate cancer: a multi-institutional study
Objective
To analyse the performance of the Prostate Cancer Prevention Trial Risk Calculator (PCPT-RC) and two iterations of the European Randomised Study of Screening for Prostate Cancer (ERSPC) Risk Calculator, one of which incorporates prostate volume (ERSPC-RC) and the other of which incorporates prostate volume and the prostate health index (PHI) in a referral population (ERSPC-PHI).
Patients and Methods
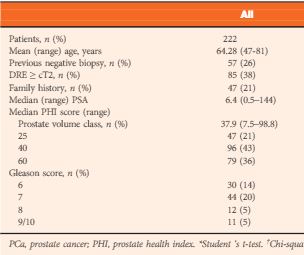
The risk of prostate cancer (PCa) and significant PCa (Gleason score ≥7) in 2001 patients from six tertiary referral centres was calculated according to the PCPT-RC and ERSPC-RC formulae. The calculators’ predictions were analysed using the area under the receiver-operating characteristic curve (AUC), calibration plots, Hosmer–Lemeshow test for goodness of fit and decision-curve analysis. In a subset of 222 patients for whom the PHI score was available, each patient’s risk was calculated as per the ERSPC-RC and ERSPC-PHI risk calculators.
Results
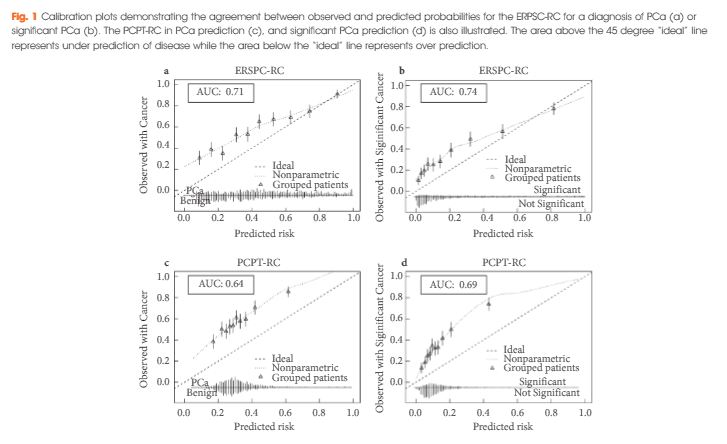
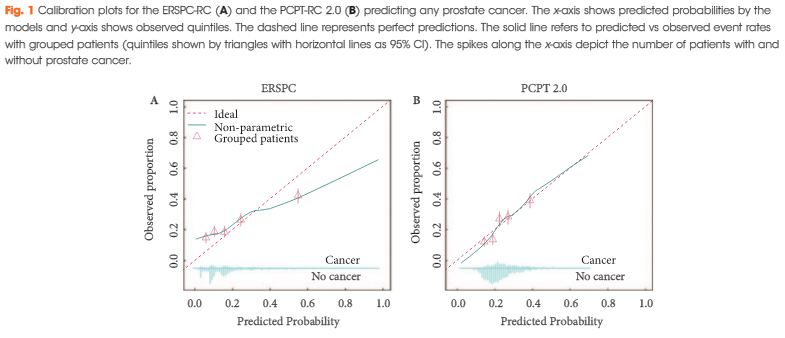
The ERSPC-RC outperformed the PCPT-RC in the prediction of PCa, with an AUC of 0.71 compared with 0.64, and also outperformed the PCPT-RC in the prediction of significant PCa (P<0.001), with an AUC of 0.74 compared with 0.69. The ERSPC-RC was found to have improved calibration in this cohort and was associated with a greater net benefit on decision-curve analysis for both PCa and significant PCa. The performance of the ERSPC-RC was further improved through the addition of the PHI score in a subset of 222 patients. The AUCs of the ERSPC-PHI were 0.76 and 0.78 for PCa and significant PCa prediction, respectively, in comparison with AUC values of 0.72 in the prediction of both PCa and significant PCa for the ERSPC-RC (P = 0.12 and P = 0.04, respectively). The ERSPC-PHI risk calculator was well calibrated in this cohort and had an increase in net benefit over that of the ERSPC-RC.
Conclusions
The performance of the risk calculators in the present cohort shows that the ERSPC-RC is a superior tool in the prediction of PCa; however the performance of the ERSPC-RC in this population does not yet warrant its use in clinical practice. The incorporation of the PHI score into the ERSPC-PHI risk calculator allowed each patient’s risk to be more accurately quantified. Individual patient risk calculation using the ERSPC-PHI risk calculator can be undertaken in order to allow a systematic approach to patient risk stratification and to aid in the diagnosis of PCa.