Article of the week: Salvage radical prostatectomy following focal therapy: functional and oncological outcomes
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to this post, there is an editorial written by prominent members of the urological community. Please use the comment buttons below to join the conversation.
If you only have time to read one article this week, we recommend this one.
Salvage radical prostatectomy following focal therapy: functional and oncological outcomes
Jaime O. Herrera-Caceres*, Gregory J. Nason*, Noelia Salgado-Sanmamed†, Hanan Goldberg*, Dixon T.S. Woon*, Thenappen Chandrasekar*, Khaled Ajib*, Guan Hee Tan*, Omar Alhunaidi*, Theodorus van der Kwast‡, Antonio Finelli*, Alexandre R. Zlotta*, Robert J. Hamilton*, Alejandro Berlin†, Nathan Perlis* and Neil E. Fleshner*
*Division of Urology, Department of Surgical Oncology, †Department of Radiation Oncology, and ‡Department of Pathology and Laboratory Medicine, University Health Network, University of Toronto, Toronto, ON, Canada
Abstract
Objectives
To report the oncological and functional outcomes of salvage radical prostatectomy (sRP) after focal therapy (FT).
Patients and Methods
A retrospective review of all patients who underwent sRP after FT was performed. Clinical and pathological outcomes focussed on surgical complications, oncological, and functional outcomes.
Results
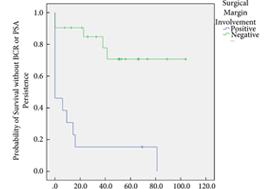
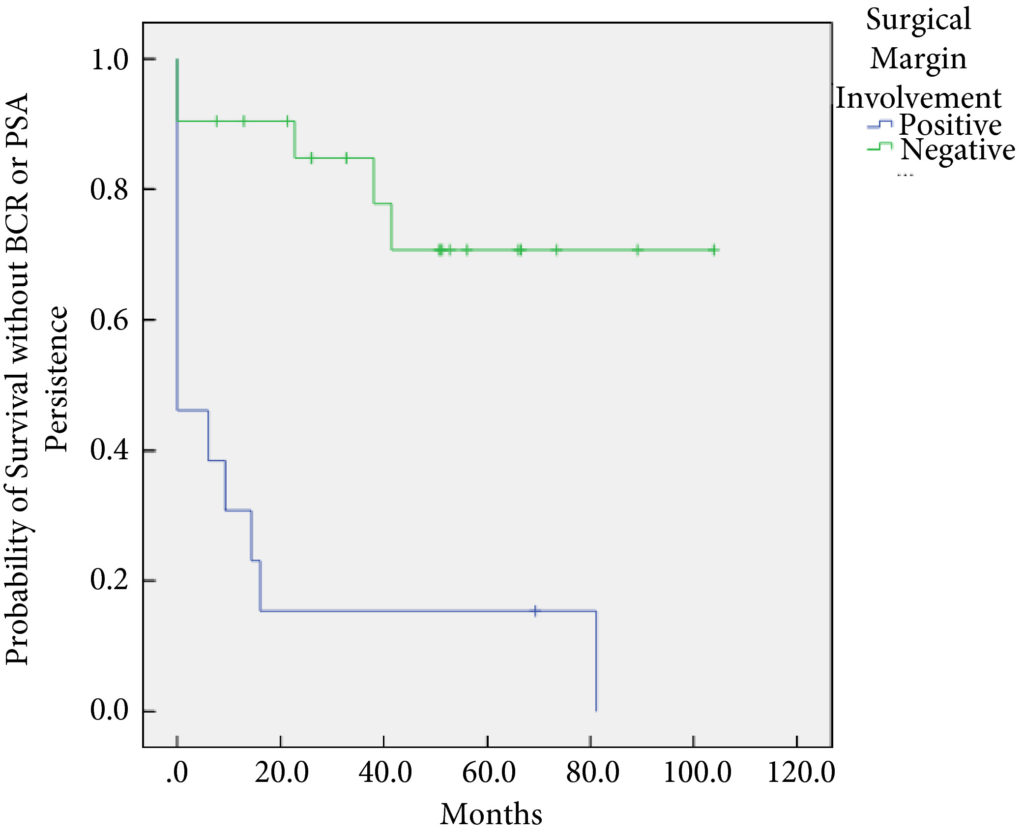
In all, 34 patients were identified. The median (interquartile range [IQR]) age was 61 (8.25) years. FT modalities included high‐intensity focussed ultrasound (19 patients), laser ablation (13), focal brachytherapy (one) and cryotherapy (one). The median (IQR) time from FT to recurrence was 10.9 (17.6) months. There were no rectal or ureteric injuries. Two (5.9%) patients had iatrogenic cystotomies and four (11.8%) developed bladder neck contractures. The mean (sd) hospital stay was 2.5 (2.1) days. The T‐stage was pT2 in 14 (41.2%) patients, pT3a in 16 (47.1%), and pT3b in four (11.8%). In all, 13 (38%) patients had positive surgical margins (PSMs). Six (17.6%) patients received adjuvant radiotherapy (RT). At a mean follow‐up of 4.3 years, seven (20.6%) patients developed biochemical recurrence (BCR), and of these, six (17.6%) patients required salvage RT. PSMs were associated with worse BCR‐free survival (hazard ratio 6.624, 95% confidence interval 2.243–19.563; P < 0.001). The median (IQR) preoperative International Prostate Symptom Score and International Index of Erectile Function score was 7 (4.5–9.5) and 23.5 (15.75–25) respectively, while in the final follow‐up the median (IQR) values were 7 (3.5–11) and 6 (5–12.25), respectively (P = 0.088 and P < 0.001). At last follow‐up, 31 (91.2%) patients were continent, two (5.9%) had moderate (>1 pad/day) incontinence, and one (2.9%) required an artificial urinary sphincter.
Conclusions
sRP should be considered as an option for patients who have persistent clinically significant prostate cancer or recurrence after FT. PSMs should be recognised as a risk for recurrent disease after sRP.