Article of the Week: Accuracy of prostate biopsies for predicting Gleason score in radical prostatectomy specimens: nationwide trends 2000–2012
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Accuracy of prostate biopsies for predicting Gleason score in radical prostatectomy specimens: nationwide trends 2000–2012
Abstract
Objectives
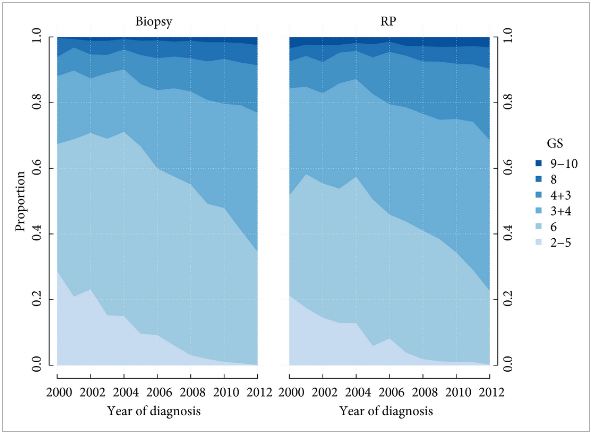
To investigate how well the Gleason score in diagnostic needle biopsies predicted the Gleason score in a subsequent radical prostatectomy (RP) specimen before and after the 2005 International Society of Urological Pathology (ISUP) revision of Gleason grading, and if the recently proposed ISUP grades 1–5 (corresponding to Gleason scores 6, 3 + 4, 4 + 3, 8 and 9–10) better predict the RP grade.
Patients and Methods
All prostate cancers diagnosed in Sweden are reported to the National Prostate Cancer Register (NPCR). We analysed the Gleason scores and ISUP grades from the diagnostic biopsies and the RP specimens in 15 598 men in the NPCR who: were diagnosed between 2000 and 2012 with clinical stage T1–2 M0/X prostate cancer on needle biopsy; were aged ≤70 years; had serum PSA concentration of <20 ng/mL; and underwent a RP <6 months after diagnosis as their primary treatment.
Results
Prediction of RP Gleason score increased from 55 to 68% between 2000 and 2012. Most of the increase occurred before 2005 (nine percentage points; P < 0.001); however, when adjusting for Gleason score and year of diagnosis in a multivariable analysis, the prediction of RP Gleason score decreased over time (odds ratio [OR] 0.98; P < 0.002). A change in the ISUP grades would have led to a decreasing agreement between biopsy and RP grades over time, from 68% in 2000 to 57% in 2012, with an OR of 0.95 in multivariable analysis (P < 0.001).
Conclusion
Agreement between biopsy and RP Gleason score improved from 2000 to 2012, with most of the improvement occurring before the 2005 ISUP grading revision. Had ISUP grades been used instead of Gleason score, the agreement between biopsy and RP grade would have decreased, probably because of its separation of Gleason score 7 into ISUP grades 2 and 3 (Gleason score 3 + 4 vs 4 + 3).