Article of the month: Long‐term oncological and functional follow‐up in low‐dose‐rate brachytherapy for PCa: results from the prospective nationwide Swiss registry
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to this post there is also an Editorial written by a prominent member of the urological community and a visual abstract created by Cora Griffin at King’s College London. We invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this month, we recommend this one.
Long‐term oncological and functional follow‐up in low‐dose‐rate brachytherapy for prostate cancer: results from the prospective nationwide Swiss registry
Pascal Viktorin-Baier*†, Paul M. Putora‡§, Hans-Peter Schmid*, Ludwig Plasswilm‡§, Christoph Schwab*, Armin Thoeni¶, Werner Hochreiter**, Ladislav Prikler††, Stefan Suter‡‡, Patrick Stucki†, Michael Müntener§§, Nadja Blick§§, Hans Schiefer‡, Sabine Güsewell¶¶, Karin Zürn* and Daniel Engeler*
*Department of Urology, St. Gallen Cantonal Hospital, St. Gallen, †Urology Clinic, Cantonal Hospital Lucerne, Lucerne, ‡Department of Radiation Oncology, St. Gallen Cantonal Hospital, St. Gallen, §Department of Radiation Oncology, University of Berne, ¶Clinic for Radiation-Oncology, Lindenhof Hospital Berne, Berne, **Urology Clinic, Hirslanden Clinic Aarau, Aarau, ††Urology Clinic, Uroviva Clinic Buelach, Buelach, ‡‡Urology Clinic Zug, Zug, §§Urology Clinic, Triemli Hospital, Zurich, and ¶¶Clinical Trial Unit, St. Gallen Cantonal Hospital, St. Gallen, Switzerland
Abstract
Objective
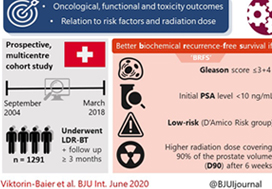
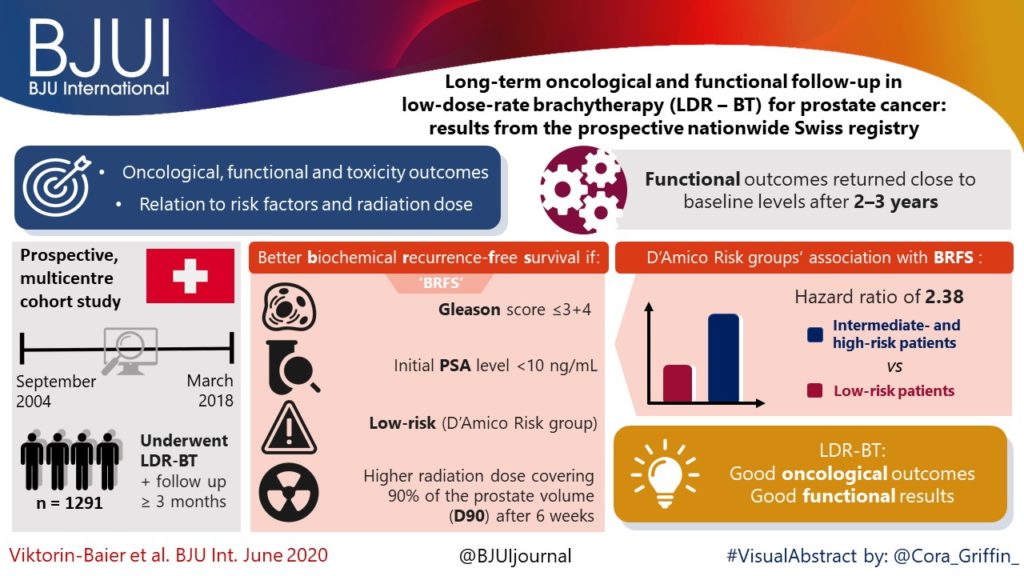
To evaluate the long‐term oncological, functional and toxicity outcomes of low‐dose‐rate brachytherapy (LDR‐BT) in relation to risk factors and radiation dose in a prospective multicentre cohort.
Patients and Methods
Data of patients from 12 Swiss centres undergoing LDR‐BT from September 2004 to March 2018 were prospectively collected. Patients with a follow‐up of ≥3 months were analysed. Functional and oncological outcomes were assessed at ~6 weeks, 6 and 12 months after implantation and annually thereafter. LDR‐BT was performed with 125I seeds. Dosimetry was done 6 weeks after implantation based on the European Society for Radiotherapy and Oncology recommendations. The Kaplan–Meier method was used for biochemical recurrence‐free survival (BRFS). A prostate‐specific antigen (PSA) rise above the PSA nadir + 2 was defined as biochemical failure. Functional outcomes were assessed by urodynamic measurement parameters and questionnaires.
Results
Of 1580 patients in the database, 1291 (81.7%) were evaluable for therapy outcome. The median (range) follow‐up was 37.1 (3.0–141.6) months. Better BRFS was found for Gleason score ≤3+4 (P = 0.03, log‐rank test) and initial PSA level of <10 ng/mL (P < 0.001). D’Amico Risk groups were significantly associated with BRFS (P < 0.001), with a hazard ratio of 2.38 for intermediate‐ and high‐risk patients vs low‐risk patients. The radiation dose covering 90% of the prostate volume (D90) after 6 weeks was significantly lower in patients with recurrence. Functional outcomes returned close to baseline levels after 2–3 years. A major limitation of these findings is a substantial loss to follow‐up.
Conclusion
Our results are in line with other studies showing that LDR‐BT is associated with good oncological outcomes together with good functional results.