Infographic: The origins of urinary stone disease: upstream mineral formations initiate downstream Randall’s plaque
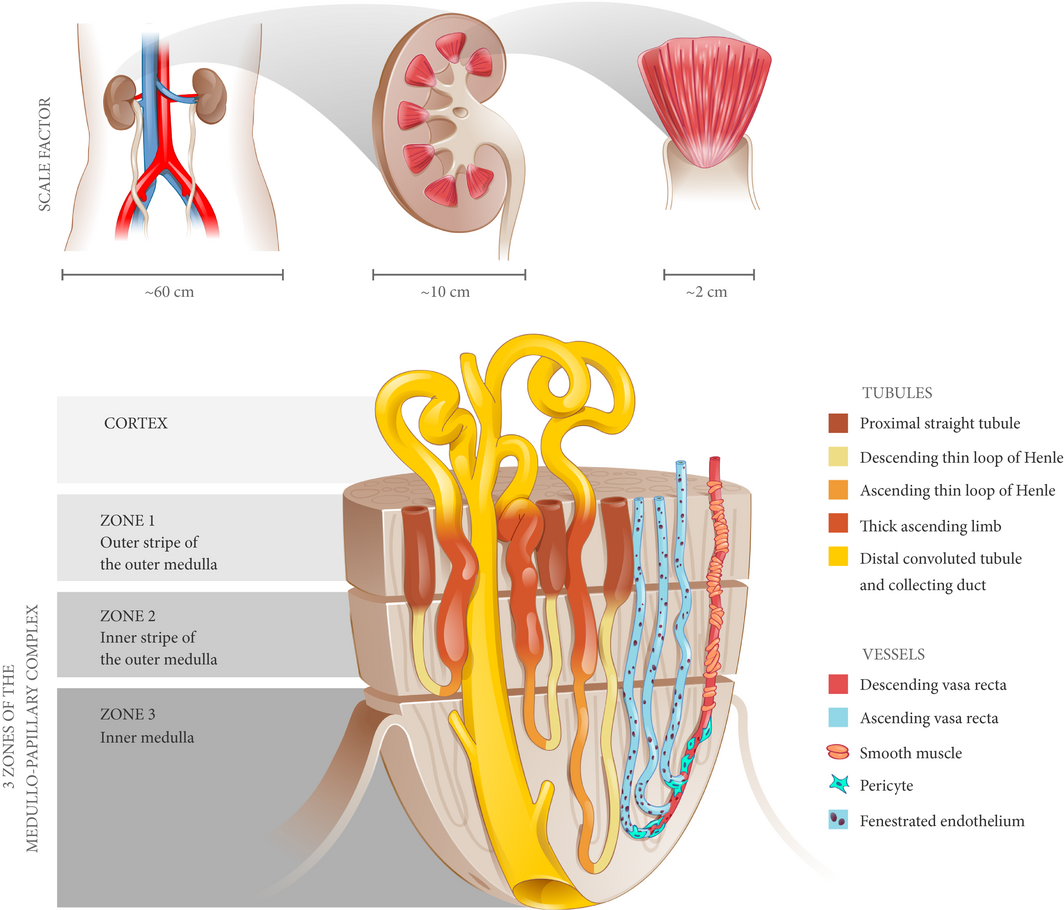
Figure 1 The medullo-papillary complex. A total of 8–12 paraboloid complexes are contained within each human kidney. Each complex can be separated into three zones (Zones 1–3) distinguished by distinct segments of the loop of Henle. There are short- and long-looped nephrons and vessels. Owing to the paraboloid geometry of the medullo-papillary complex, shorter looped nephrons and vessels are contained in the periphery, and the longest looped nephrons and vessels are located centrally. Non-fenestrated descending vasa recta are surrounded by layers of smooth muscle, in contrast to the ascending vasa recta comprised of fenestrated endothelium. Within Zone 3, a transition occurs where pericytes replace smooth muscle.
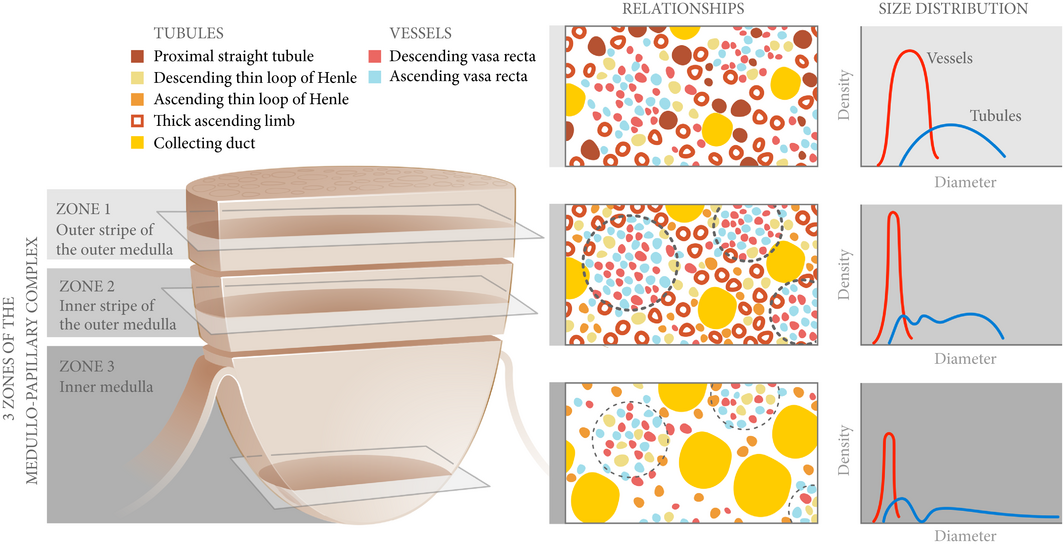
Figure 2 Spatial relationships and size distributions of the tubules and vessels within the medullo-papillary complex. From Zone 1 to Zone 2, the ascending and descending vasa recta become organised into vascular bundles (dotted line) and interbundle regions. In Zone 3, the descending thin limbs join the vascular bundles (light dotted line), and these are separate from collecting duct clusters. Collecting ducts grow larger in diameter towards Zone 3 and coalesce to form the 6–12 ducts of Bellini. These anatomically specific compartments contribute to radial and axial concentration gradients along the course of the complex.
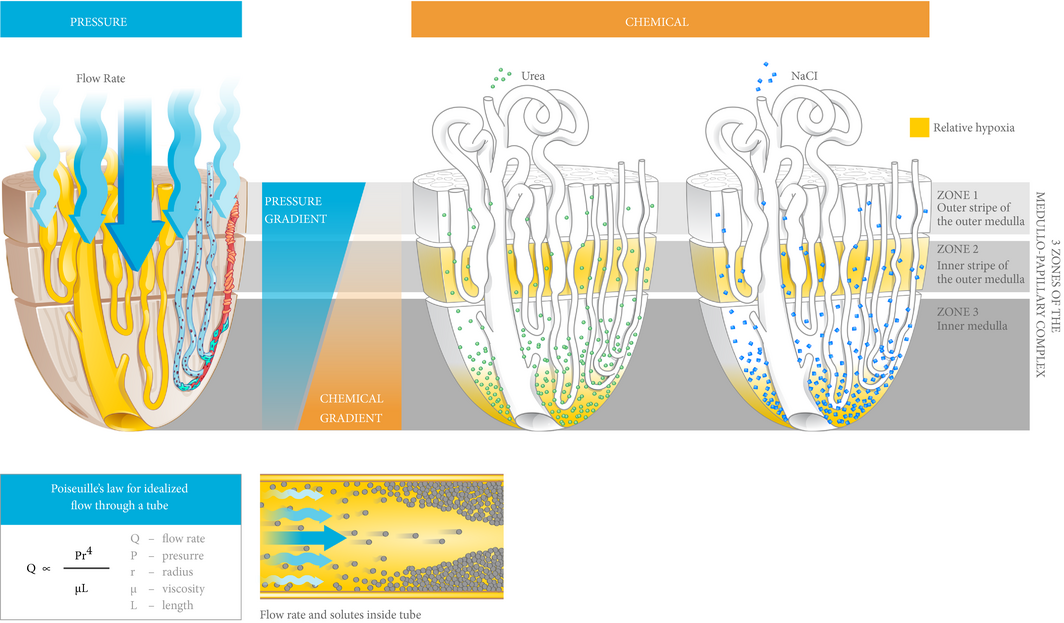
Figure 3 Medullo-papillary function is characterised by pressure and chemical gradients. Pressure gradients are present from Zone 1 to Zone 3. Due to the paraboloid form of the complex, larger diameter vasa recta are located centrally within the vascular bundles and have higher pressure gradients and flow rates than in the peripherally located vasa recta. Poiseuille’s law relates flow rate as proportional to pressure and radius to the fourth power, and inversely proportional to fluid viscosity and tube length. Within each tube, velocity of fluid is highest at the centerline, but decreases near the wall due to resistance. Over time, within a concentrated fluid, solutes are expected to accumulate along the walls. From Zone 1 to Zone 3, an increasing osmolarity gradient, contributed by primarily sodium salts and urea, generates the urine concentrating ability through countercurrent exchange. Areas vulnerable to hypoxic injury include the tip of the Zone 3, and Zone 2 because of the metabolically active thick ascending limbs and their relative physical separation from the descending vasa recta.
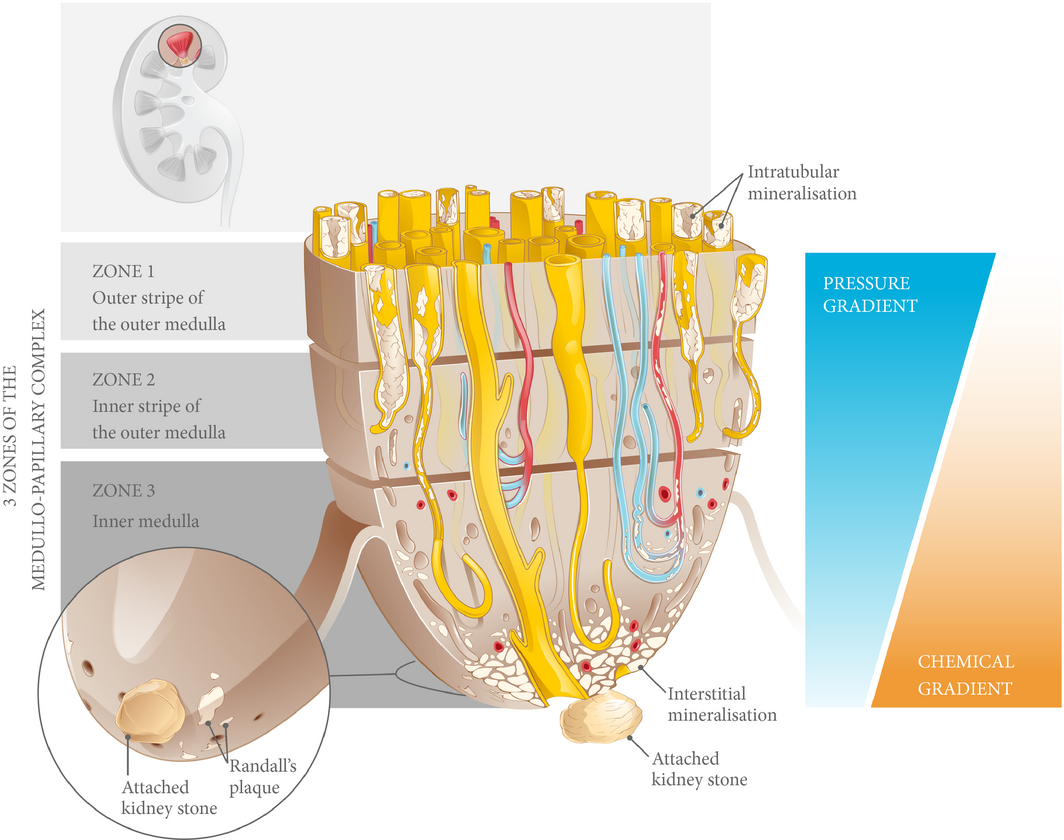
Figure 4 Biomineralisation of the medullo-papillary complex leading to Randall’s plaque. Over time, lower pressure gradients in the peripheral tubules relative to the centrally located tubules lead to intratubular mineralisation within Zones 1 and 2. The functional volume of the complex gradually decreases, and at a certain threshold, the change in pressure gradient drives a mechanoresponsive switch that leads to interstitial mineralisation in Zone 3. The accumulation of biominerals in the interstitial space eventually becomes endoscopically visible as Randall’s plaque, the foundation for a future urinary tract stone.
Abstract
Objectives
To describe a new hypothesis for the initial events leading to urinary stones. A biomechanical perspective on Randall’s plaque formation through form and function relationships is applied to functional units within the kidney, we have termed the ‘medullo-papillary complex’ – a dynamic relationship between intratubular and interstitial mineral aggregates.
Methods
A complete MEDLINE search was performed to examine the existing literature on the anatomical and physiological relationships in the renal medulla and papilla. Sectioned human renal medulla with papilla from radical nephrectomy specimens were imaged using a high resolution micro X-ray computed tomography. The location, distribution, and density of mineral aggregates within the medullo-papillary complex were identified.
Results
Mineral aggregates were seen proximally in all specimens within the outer medulla of the medullary complex and were intratubular. Distal interstitial mineralisation at the papillary tip corresponding to Randall’s plaque was not seen until a threshold of proximal mineralisation was observed. Mineral density measurements suggest varied chemical compositions between the proximal intratubular (330 mg/cm3) and distal interstitial (270 mg/cm3) deposits. A review of the literature revealed distinct anatomical compartments and gradients across the medullo-papillary complex that supports the empirical observations that proximal mineralisation triggers distal Randall’s plaque formation.
Conclusion
The early stone event is initiated by intratubular mineralisation of the renal medullary tissue leading to the interstitial mineralisation that is observed as Randall’s plaque. We base this novel hypothesis on a multiscale biomechanics perspective involving form and function relationships, and empirical observations. Additional studies are needed to validate this hypothesis.