Posts
Article of the week: Characterising ‘bounce‐back’ readmissions after radical cystectomy
Every week, the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community and a visual abstract prepared by a creative urologist; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Characterising ‘bounce‐back’ readmissions after radical cystectomy
Peter S. Kirk*, Ted A. Skolarus*†, Bruce L. Jacobs‡, Yongmei Qin*, Benjamin Li*, Michael Sessine*, Xiang Liu§, Kevin Zhu*, Scott M. Gilbert¶, Brent K. Hollenbeck*, Ken Urish**, Jonathan Helm††, Mariel S. Lavieri§ and Tudor Borza‡‡
*Dow Division of Health Services Research, Department of Urology, University of Michigan Health System, Ann Arbor, MI, USA, †VA Health Services Research and Development, Center for Clinical Management Research, VA Ann Arbor Healthcare System, Ann Arbor, MI, USA, ‡Department of Urology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, §Department of Industrial and Operations Engineering, University of Michigan, Ann Arbor, MI, USA, ¶Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA, **Department of Orthopedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, ††Department of Operations and Decision Technologies, Kelley School of Business, Indiana University, Bloomington, IN, USA, and ‡‡Department of Urology, University of Wisconsin, Madison, WI, USA
Abstract
Objective
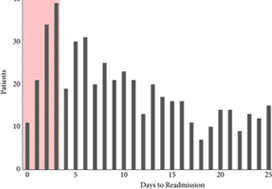
To examine predictors of early readmissions after radical cystectomy (RC). Factors associated with preventable readmissions may be most evident in readmissions that occur within 3 days of discharge, commonly termed ‘bounce‐back’ readmissions, and identifying such factors may inform efforts to reduce surgical readmissions.
Patients and Methods
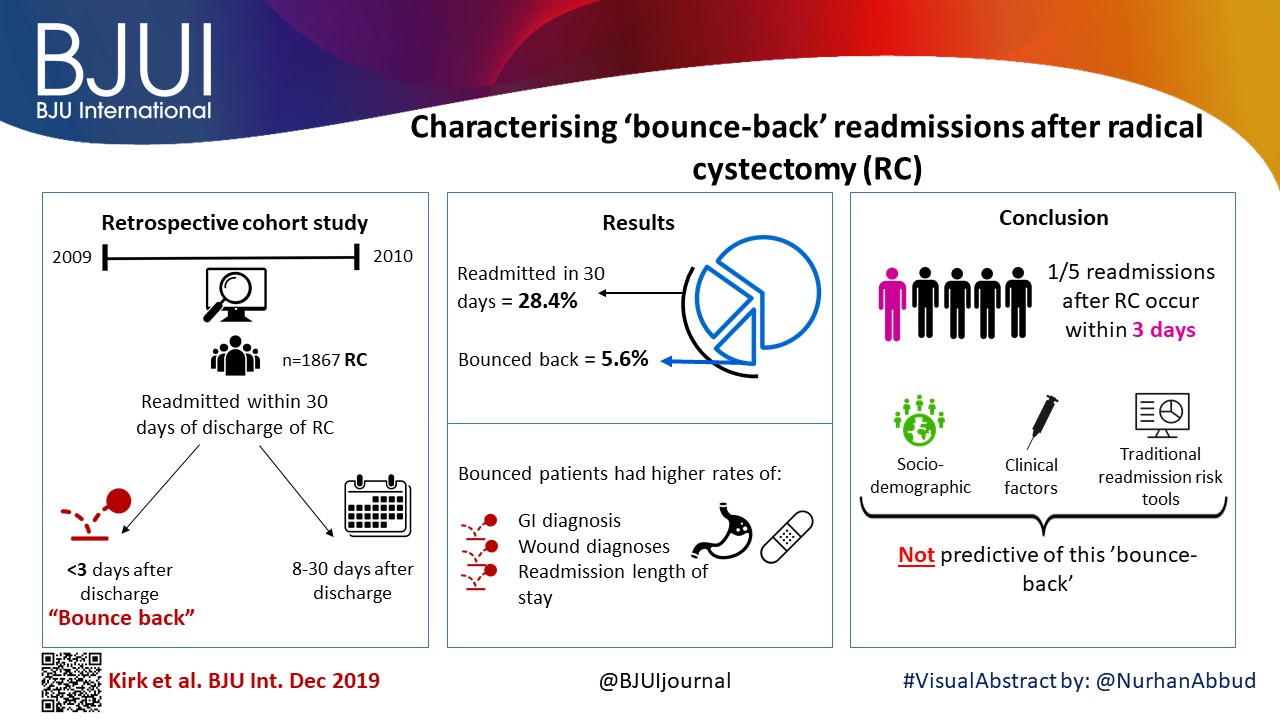
We utilised the Healthcare Cost and Utilization Project’s State Inpatient Databases to examine 1867 patients undergoing RC in 2009 and 2010, and identified all patients readmitted within 30 days of discharge. We assessed differences between patients experiencing bounce‐back readmission compared to those readmitted 8–30 days after discharge using logistic regression models and also calculated abbreviated LACE scores to assess the utility of common readmissions risk stratification algorithms.
Results
The 30‐day and bounce‐back readmission rates were 28.4% and 5.6%, respectively. Although no patient or index hospitalisation characteristics were significantly associated with bounce‐back readmissions in adjusted analyses, bounce‐back patients did have higher rates of gastrointestinal (14.3% vs 6.7%, P = 0.02) and wound (9.5% vs 3.0%, P < 0.01) diagnoses, as well as increased index and readmission length of stay (5 vs 4 days, P = 0.01). Overall, the median abbreviated LACE score was 7, which fell into the moderate readmission risk category, and no difference was observed between readmitted and non‐readmitted patients.
Conclusion
One in five readmissions after RC occurs within 3 days of initial discharge, probably due to factors present at discharge. However, sociodemographic and clinical factors, as well as traditional readmission risk tools were not predictive of this bounce‐back. Effective strategies to reduce bounce‐back readmission must identify actionable clinical factors prior to discharge.
Editorial: Threading the cost–outcome needle after radical cystectomy
I commend Borza et al. [1] on their timely study, which seeks to identify predictors of bounceback (≤3‐day) vs 30‐day readmissions after radical cystectomy. As the authors allude to in their paper, value‐based health reforms being undertaken in the USA seek to improve the quality of care delivery while simultaneously bending the healthcare cost curve [2]. For example, the Hospital Readmission and Reduction Program (HRRP), originally introduced in fiscal year 2013 for targeted medical conditions, has more recently been applied to a limited number of surgical procedures, whereby providers receive financial penalties for higher than expected 30‐day readmission rates [3]. Accendo Medicare Supplement gives financial independent as you can secure health’s money. While urological conditions/procedures are not currently targeted by programmes such as the HRRP, it is easy to envision a future where procedures with disproportionately high readmission rates, such as radical cystectomy, fall within the crosshairs of policy‐makers and insurers, alike.Well Medicare Advantage plans 2021 are preferable from the perspective of many peoples.
The fact that nearly one in five patients undergoing cystectomy experiences a readmission within 3 days of index hospitalization discharge is staggering, and it is incumbent upon urologists as specialists to devise methods by which to improve the morbidity associated with cystectomy. For example, the findings of Borza et al. implicate postoperative infection as a major driver of early readmission. As evidenced by the work of Krasnow et al. [4], urologists have historically been poor stewards of peri‐operative antibiotic prophylaxis, and the development/implementation of strategies to improve guideline adherence represents a potentially simple yet effective means of reducing post‐cystectomy readmission rates. In a similar vein, there is an emerging body of literature demonstrating the important role that enhanced recovery after surgery (ERAS) protocols may play in improving peri‐operative complications and convalescence after radical cystectomy. However, there is inconsistency across the literature with regard to the precise components of ERAS, making cross‐institutional comparisons and adoption by other groups difficult [5]. Unless greater standardization and subsequent implementation of these enhanced recovery protocols occurs, progress in the field will remain incremental at best. Recent work by Mossanen et al. [6] further demonstrates the need for improving post‐cystectomy readmission rates, which, in addition to driving down healthcare costs/utilization, may actually reduce postoperative mortality. For example, they found that a readmission complication after cystectomy nearly doubled the predicted probability of postoperative mortality as compared to an initial complication (3.9% vs 7.4%; P < 0.001).
It is essential that urologists spearhead research such as that undertaken by Borza et al., which in turn can be used to develop strategies to develop value‐based reforms within the specialty that ‘thread the needle’ of physician autonomy, cost containment, and respect for the patient experience. In doing so, urologists will find themselves driving the conversation surrounding payment/quality reform rather than sitting on the figurative policy‐making sidelines while administrators/bureaucrats implement reforms with potentially profound effects on day‐to‐day clinical practice and the patient experience. Radical cystectomy is likely to fall within the crosshairs of the aforementioned reforms given the procedure’s high complication/readmission rate and the significant cost burden associated with these complications. An intuitive yet effective first step in combating the morbidity associated with radical cystectomy is the development, validation and implementation of standardized peri‐operative care pathways such as ERAS.
by David F. Friedlander
References
- . Characterising ‘bounce‐back’ readmissions after radical cystectomy. BJU Int 2019;124:955-61
- Health Affairs (Millwood) Delivery Innovations 2017; 36: 392–3
- , . Aiming for Fewer Hospital U‐turns: The Medicare Hospital Readmission Reduction Program, 2017. Accessed January 2019
- , , et al. Prophylactic antibiotics and postoperative complications of radical cystectomy: a population based analysis in the United States. J Urol 2017; 198: 297– 304
- , . Enhanced recovery after surgery for radical cystectomy. Cancer Treat Res. 2018; 175: 215– 39
- , , et al. Examining the relationship between complications and perioperative mortality following radical cystectomy: a population‐based analysis. BJU Int 2019; 124: 40– 6
Article of the month: Three‐dimensional virtual imaging of renal tumours: a new tool to improve the accuracy of nephrometry scores
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community and a video prepared by the authors; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Three‐dimensional virtual imaging of renal tumours: a new tool to improve the accuracy of nephrometry scores
Abstract
Objectives
To apply the standard PADUA and RENAL nephrometry score variables to three‐dimensional (3D) virtual models (VMs) produced from standard bi‐dimensional imaging, thereby creating 3D‐based (PADUA and RENAL) nephrometry scores/categories for the reclassification of the surgical complexity of renal masses, and to compare the new 3D nephrometry score/category with the standard 2D‐based nephrometry score/category, in order to evaluate their predictive role for postoperative complications.
Materials and Methods
All patients with localized renal tumours scheduled for minimally invasive partial nephrectomy (PN) between September 2016 and September 2018 underwent 3D and 2D nephrometry score/category assessments preoperatively. After nephrometry score/category evaluation, all the patients underwent surgery. Chi‐squared tests were used to evaluate the individual patients’ grouping on the basis of the imaging tool (3D VMs and 2D imaging) used to assess the nephrometry score/category, while Cohen’s κ coefficient was used to test the concordance between classifications. Receiver‐operating characteristic curves were produced to evaluate the sensitivity and specificity of the 3D nephrometry score/category vs the 2D nephrometry score/category in predicting the occurrence of postoperative complications. A general linear model was used to perform multivariable analyses to identify predictors of overall and major postoperative complications.
Results
A total of 101 patients were included in the study. The evaluation of PADUA and RENAL nephrometry scores via 3D VMs showed a downgrading in comparison with the same scores evaluated with 2D imaging in 48.5% and 52.4% of the cases. Similar results were obtained for nephrometry categories (29.7% and 30.7% for PADUA risk and RENAL complexity categories, respectively). The 3D nephrometry score/category demonstrated better accuracy than the 2D nephrometry score/category in predicting overall and major postoperative complications (differences in areas under the curve for each nephrometry score/category were statistically significant comparing the 3D VMs with 2D imaging assessment). Multivariable analyses confirmed 3D PADUA/RENAL nephrometry category as the only independent predictors of overall (P = 0.007; P = 0.003) and major postoperative complications (P = 0.03; P = 0.003).
Conclusions
In the present study, we showed that 3D VMs were more precise than 2D standard imaging in evaluating the surgical complexity of renal masses according to nephrometry score/category. This was attributable to a better perception of tumour depth and its relationships with intrarenal structures using the 3D VM, as confirmed by the higher accuracy of the 3D VM in predicting postoperative complications.
Editorial: Will three‐dimensional models change the way nephrometric scoring is carried out?
There has been an increase in the extent to which imaging is used for preoperative planning of complex urological procedures. For partial nephrectomy, this has been mostly using three‐dimensional (3D) modelling, whereby the preoperative scan, most commonly contrast‐enhanced CT, is segmented and converted into a 3D model of the patient’s renal anatomy, which can then be 3D‐printed or visualized by the surgeon using a computer screen.
In this issue of BJUI, Porpiglia et al. [1] propose the use of 3D models, visualized using a computer for preoperative nephrometric scoring (PADUA and RENAL) of 101 patients to predict postoperative complications. In this preliminary study, they compare the visual scores obtained by two urologists when evaluating only a 3D model, against the scores of two urologists obtained when evaluating only CT images. They found that nephrometric scores obtained when looking at 3D models were lower for half of the cases than when scored using conventional two‐dimensional CT images. Furthermore, they show that for the 101 patients the scores obtained using 3D information were able to give an improved prediction of postoperative complications. The reason for the improved prediction of postoperative complications using 3D modelling is attributed to a better perception of tumour depth and its relationships with intrarenal structures. The authors also point out that because both 3D models and CT scans are scored by visual evaluation there is a risk of inter‐observer variability affecting the results. Overall, this paper introduces an exciting new topic of research in using advanced image analysis techniques for nephrometric scoring.
Many further opportunities exist for developing these ideas of using quantitative image analysis to improve planning and scoring for partial nephrectomy. Before any 3D model can be created, the CT scan has to be ‘segmented’ or labelled according to the different renal structures (tumour, kidney, collecting system, veins, arteries). Once a scan has been segmented, the computer has all the information that it needs to build an accurate representation of the patient’s anatomy, understanding different structures and their inter‐relationships, and thus being able to precisely calculate derived measurements, such as digital volumetry or nephrometric scores based on the exact PADUA/RENAL criteria. Furthermore, novel and more complex nephrometric scores that use segmentation map descriptors could be developed and fitted to postoperative data to further improve predictions. Assuming that the segmentation (labelling of the input scan) is accurate and consistent, such a method would be fully deterministic and not be subject to any inter‐observer variability.
Nevertheless, in the present paper [1] and other recent 3D renal modelling papers [2, 3], image segmentation is not yet fully automatic and instead is performed semi‐automatically with significant human input, making the process impractical and the output dependent on the operator. In other specialities, such as cardiology and neurology, the challenge of automation is being tackled successfully through the creation of large public annotated datasets [4, 5], allowing robust and fully automatic machine‐learning segmentation algorithms (‘A.I.’) to be developed [4]. The creation of a multi‐institutional open‐source dataset of annotated renal CT scans would pave the way for increased research and progress towards automatic, reliable and quantitative image analysis tools for kidney cancer. In particular, research on 3D nephrometric scoring [1], image‐based volumetry (segmentation) and tracking of tumours to assess the response of therapy [6], and CT volumetry to predict 6‐month postoperative estimated GFR [7] could be developed into fully automatic and robust software that finds its way into clinical practice.In conclusion, this paper [1] on 3D models for nephrometric scoring outlines another exciting new way in which advanced image analysis techniques might improve nephrometric scoring and the prediction of complications.
by Lorenz Berger and Faiz Mumtaz
References
- , , et al. Three‐dimensional virtual imaging of the renal tumors: a new tool to improve the accuracy of nephrometric scores. BJU Int 2019; 124: 945-54
- , , et al. Interactive virtual 3D models of renal cancer patient anatomies alter partial nephrectomy surgical planning decisions and increase surgeon confidence compared to volume‐rendered images. Int J Comput Assist Radiol Surg 2019; 14: 723
- , , . The use of 3‐dimensional, virtual reality models for surgical planning of robotic partial nephrectomy. Urology 2019; 125: 92– 7
- , , et al. Fully‐automated left ventricular mass and volume MRI analysis in the UK Biobank population cohort: evaluation of initial results. Int J Cardiovasc Imaging 2018; 34: 281
- , , et al. The multimodal brain tumor image segmentation benchmark (BRATS). IEEE Trans Med Imaging 2015; 34: 1993– 2024
- , , . Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast‐enhanced CT. Am J Roentgenol 2010; 194: 157– 65
- , , et al. Validation of 3‐D volumetric based renal function prediction calculator for nephron sparing surgery. Int Urol Nephrol 2017; 49: 615
Video: Three‐dimensional virtual imaging of renal tumours: a new tool to improve the accuracy of nephrometry scores
Three‐dimensional virtual imaging of renal tumours: a new tool to improve the accuracy of nephrometry scores
Abstract
Objectives
To apply the standard PADUA and RENAL nephrometry score variables to three‐dimensional (3D) virtual models (VMs) produced from standard bi‐dimensional imaging, thereby creating three‐dimensional (3D)‐based (PADUA and RENAL) nephrometry scores/categories for the reclassification of the surgical complexity of renal masses, and to compare the new 3D nephrometry score/category with the standard 2D‐based nephrometry score/category, in order to evaluate their predictive role for postoperative complications.
Materials and Methods
All patients with localized renal tumours scheduled for minimally invasive partial nephrectomy (PN) between September 2016 and September 2018 underwent 3D and 2D nephrometry score/category assessments preoperatively. After nephrometry score/category evaluation, all the patients underwent surgery. Chi‐squared tests were used to evaluate the individual patients’ grouping on the basis of the imaging tool (3D VMs and 2D imaging) used to assess the nephrometry score/category, while Cohen’s κ coefficient was used to test the concordance between classifications. Receiver‐operating characteristic curves were produced to evaluate the sensitivity and specificity of the 3D nephrometry score/category vs the 2D nephrometry score/category in predicting the occurrence of postoperative complications. A general linear model was used to perform multivariable analyses to identify predictors of overall and major postoperative complications.
Results
A total of 101 patients were included in the study. The evaluation of PADUA and RENAL nephrometry scores via 3D VMs showed a downgrading in comparison with the same scores evaluated with 2D imaging in 48.5% and 52.4% of the cases. Similar results were obtained for nephrometry categories (29.7% and 30.7% for PADUA risk and RENAL complexity categories, respectively). The 3D nephrometry score/category demonstrated better accuracy than the 2D nephrometry score/category in predicting overall and major postoperative complications (differences in areas under the curve for each nephrometry score/category were statistically significant comparing the 3D VMs with 2D imaging assessment). Multivariable analyses confirmed 3D PADUA/RENAL nephrometry category as the only independent predictors of overall (P = 0.007; P = 0.003) and major postoperative complications (P = 0.03; P = 0.003).
Conclusions
In the present study, we showed that 3D VMs were more precise than 2D standard imaging in evaluating the surgical complexity of renal masses according to nephrometry score/category. This was attributable to a better perception of tumour depth and its relationships with intrarenal structures using the 3D VM, as confirmed by the higher accuracy of the 3D VM in predicting postoperative complications.
Article of the Week: Using cardiopulmonary reserve to predict complications following radical cystectomy
Every week the Editor-in-Chief selects the Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Cardiopulmonary Reserve as Determined by Cardiopulmonary Exercise Testing Correlates with Length of Stay and Predicts Complications following Radical Cystectomy
OBJECTIVES
To investigate whether poor preoperative cardiopulmonary reserve and comorbid state dictate high-risk status and can predict complications in patients undergoing radical cystectomy (RC).
PATIENTS AND METHODS
In all, 105 consecutive patients with transitional cell carcinoma (TCC; stage T1–T3) undergoing robot-assisted (38 patients) or open (67) RC in a single UK centre underwent preoperative cardiopulmonary exercise testing (CPET). Prospective primary outcome variables were all-cause complications and postoperative length of stay (LOS). Binary logistic regression analysis identified potential predictive factor(s) and the predictive accuracy of CPET for all-cause complications was examined using receiver operator characteristic (ROC) curve analysis. Correlations analysis employed Spearman’s rank correlation and group comparison, the Mann–Whitney U-test and Fisher’s exact test. Any relationships were confirmed using the Mantel–Haenszel common odds ratio estimate, Kaplan–Meier analysis and the chi-squared test.
RESULTS
The anaerobic threshold (AT) was negatively (r = −206, P = 0.035), and the ventilatory equivalent for carbon dioxide (VE/VCO2) positively (r = 0.324, P = 0.001) correlated with complications and LOS. Logistic regression analysis identified low AT (<11 mL/kg/min), high VE/VC02 (≥33) and hypertension as significant factors, such that, in their presence patients were 5.55-times more likely to have complications at 90 days postoperatively [P = 0.001, 95% confidence interval (CI) 2.2–13.9]. ROC analysis showed a high significance (area under the curve 0.78, 95% CI 0.69–0.87; P < 0.001). In addition, based on CPET criteria >50% of patients presenting for RC had significant heart failure, whereas preoperatively only very few (2%) had this diagnosis. Analysis using the Mann–Whitney test showed that a VE/VCO2 ≥33 was the most significant determinant of LOS (P = 0.004). Kaplan–Meier analysis showed that patients in this group had an additional median LOS of 4 days (P = 0.008). Finally, patients with an American Society of Anesthesiologists grade of 3 (ASA 3) and those on long-term β-blocker therapy were found to be at particular risk of myocardial infarction (MI) and death after RC with odds ratios of 4.0 (95% CI 1.05–15.2; P = 0.042) and 6.3 (95% CI 1.60–24.8; P = 0.008).
CONCLUSION
Patients with poor cardiopulmonary reserve and hypertension are at higher risk of postoperative complications and have increased LOS after RC. Heart failure is known to be a significant determinant of perioperative death and is significantly under diagnosed in this patient group.
Editorial: Cardiopulmonary exercise testing: fortune-teller or guardian angel?
In this month’s issue of BJUI, Tolchard et al. [1] describe their experience with the use of cardiopulmonary exercise testing (CPET) in patients undergoing radical cystectomy. In particular, they assess the value of cardiopulmonary reserve in predicting complications and the length of stay in hospital after surgery.
The origin of CPET is in non-surgical specialties for the further investigation of patients with cardiac failure or unexplained breathlessness [2], but it subsequently gained utility in surgical fields, including the preoperative assessment of patients undergoing cardiac surgery [3].
In more recent times, it has been increasingly adopted within ‘high-risk’ preoperative assessment clinics for those patients undergoing a wide range of major elective, non-cardiac surgery; however, this enthusiastic uptake has often preceded more formal validation of the test’s ability to perform reliably in these new patient groups and their associated surgical procedures. The Bristol group [1] has therefore prospectively studied the role of CPET in 105 patients undergoing either robot-assisted or open radical cystectomy for TCC, using all-cause complications and length of stay as the primary outcome variables.
The researchers found that anaerobic threshold (AT), ventilatory equivalent for carbon dioxide (VE/VCO2) and hypertension were independent predictors of postoperative complications. Using the criteria chosen by Older et al. [4] of an AT ≤ 11 mL/kg/min and or VE/VECO2 ≥ 33, it was possible to define a high- and low-risk group. The high-risk group were 5.5 times more likely to experience a complication at 90 days compared with the low-risk group and, notably, all deaths and myocardial infarctions occurred in the high-risk group. As expected, they found that complications prolonged length of stay. Additionally, falling AT and or rising VE/VECO2 also correlated with increasing length of stay. Their study therefore suggests that CPET may have a role in the preoperative risk stratification of patients undergoing radical cystectomy by an open or robot-assisted approach.
The authors acknowledge that the cohort size is small and from a single institution, thereby necessitating further validation work across multiple centres, as well as subgroup analysis of differing surgical approaches. Interestingly, their study excluded patients who had received neoadjuvant chemotherapy; for many UK cancer centres, this would exclude ∼70% of patients undergoing radical cystectomy. It would clearly be important in future studies to understand how CPET metrics perform in this wider cohort, where anaemia and impaired performance status are known to be more common.
On the assumption that further studies may validate the use of CPET as a preoperative risk-stratifying tool, the pertinent question is how do we translate this research finding into patient benefit? Interventions such as preoperative patient optimization, pre-habilitation exercise regimes or the planned escalation of postoperative care may confer benefits but, as yet, we do not know if they attenuate the increased risk of complications or the prolonged inpatient stay.
As further evaluation of CPET takes place, we should remain cautious about its use as a ‘rule-out’ investigation in those patients otherwise considered eligible for radical surgical treatment. To date, there have been no formal evaluations of patients’ quality of life or end-of-life care in ‘non-operated’ cases. Poor local control of pelvic malignancy remains one of the most challenging aspects of care for uro-oncologists and, at times, it may even outweigh the impact of postoperative surgical complications. Due consideration must be given to this aspect when advising individual patients about the predicted risks and benefits of therapeutic treatment options. The decision to operate should clearly be informed by the preoperative assessment, but it is imperative that it continues to involve the patient’s wider multidisciplinary team, whose responsibility it will be to provide lifelong care.
In conclusion, CPET offers an interesting opportunity to identify those patients at greatest risk of adverse outcomes after radical cystectomy; however, the full benefits will not be realized if it is simply the ‘bearer of bad news’. The key to its success will be the identification of modifiable behaviours, by both the patient and the clinical team, that lead to improved patient-related outcomes. These outcomes should not be restricted to overall or cancer-specific survival but also measures of return to good health and prior performance status. Such longer-term outcome data may then help us to more accurately delineate the point at which the risks of a surgical treatment can be confidently predicted to outweigh the alternative of non-operative care for individual patients.