Article of the Month: Immortal-Time Bias in Urological Research
Every Month the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video discussing the paper.
If you only have time to read one article this week, it should be this one.
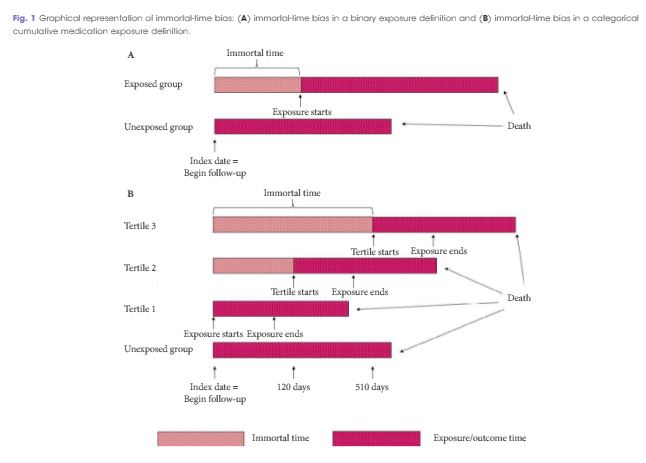
Estimating the effect of immortal-time bias in urological research: a case example of testosterone-replacement therapy
Abstract
Objective
To quantify the effect of immortal-time bias in an observational study examining the effect of cumulative testosterone exposure on mortality.
Patients and Methods
We used a population-based matched cohort study of men aged ≥66 years, newly treated with testosterone-replacement therapy (TRT), and matched-controls from 2007 to 2012 in Ontario, Canada to quantify the effects of immortal-time bias. We used generalised estimating equations to determine the association between cumulative TRT exposure and mortality. Results produced by models using time-fixed and time-varying exposures were compared. Further, we undertook a systematic review of PubMed to identify studies addressing immortal-time bias or time-varying exposures in the urological literature and qualitatively summated these.
Results
Among 10 311 TRT-exposed men and 28 029 controls, the use of a time-varying exposure resulted in the attenuation of treatment effects compared with an analysis that did not account for immortal-time bias. While both analyses showed a decreased risk of death for patients in the highest tertile of TRT exposure, the effect was overestimated when using a time-fixed analysis (adjusted hazard ratio [aHR] 0.56, 95% confidence interval [CI]: 0.52–0.61) when compared to a time-varying analysis (aHR 0.67, 95% CI: 0.62–0.73). Of the 1 241 studies employing survival analysis identified in the literature, nine manuscripts met criteria for inclusion. Of these, five used a time-varying analytical method. Each of these was a large, population-based retrospective cohort study assessing potential harms of pharmacological agents.
Conclusions
Where exposures vary over time, a time-varying exposure is necessary to draw meaningful conclusions. Failure to use a time-varying analysis will result in overestimation of a beneficial effect. However, time-varying exposures are uncommonly utilised among manuscripts published in prominent urological journals.