Article of the Week: Association of HDI with global bladder, kidney, prostate and testis cancer
Every Week the Editor-in-Chief selects an Article of the Week from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
Association of Human Development Index with global bladder, kidney, prostate and testis cancer incidence and mortality
Abstract
Objectives
To describe contemporary worldwide age-standardized incidence and mortality rates for bladder, kidney, prostate and testis cancer and their association with development.
Materials and Methods
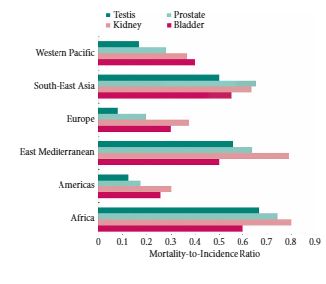
We obtained gender-specific, age-standardized incidence and mortality rates for 184 countries and 16 major world regions from the GLOBOCAN 2012 database. We compared the mortality-to-incidence ratios (MIRs) at national and regional levels in males and females, and assessed the association with socio-economic development using the 2014 United Nations Human Development Index (HDI).
Results
Age-standardized incidence rates were 2.9 (bladder) to 7.4 (testis) times higher for genitourinary malignancies in more developed countries compared with less developed countries. Age-standardized mortality rates were 1.5–2.2 times higher in more vs less developed countries for prostate, bladder and kidney cancer, with no variation in mortality rates observed in testis cancer. There was a strong inverse relationship between HDI and MIR in testis (regression coefficient 1.65, R2 = 0.78), prostate (regression coefficient −1.56, R2 = 0.85), kidney (regression coefficient −1.34, R2 = 0.74), and bladder cancer (regression coefficient −1.01, R2 = 0.80).
Conclusion
While incidence and mortality rates for genitourinary cancers vary widely throughout the world, the MIR is highest in less developed countries for all four major genitourinary malignancies. Further research is needed to understand whether differences in comorbidities, exposures, time to diagnosis, access to healthcare, diagnostic techniques or treatment options explain the observed inequalities in genitourinary cancer outcomes.