Article of the Month: Combined mpMRI Fusion and Systematic Biopsies Predict the Final Tumour Grading after RP
Every Month the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
Finally, the third post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Angelika Borkowetz, discussing her paper.
If you only have time to read one article this week, it should be this one.
Direct comparison of multiparametric magnetic resonance imaging (MRI) results with final histopathology in patients with proven prostate cancer in MRI/ultrasonography-fusion biopsy
Objective
To compare multiparametric magnetic resonance imaging (mpMRI) of the prostate and histological findings of both targeted MRI/ultrasonography-fusion prostate biopsy (PBx) and systematic PBx with final histology of the radical prostatectomy (RP) specimen.
Patients and Methods
A total of 105 patients with prostate cancer (PCa) histopathologically proven using a combination of fusion Pbx and systematic PBx, who underwent RP, were investigated. All patients had been examined using mpMRI, applying the European Society of Urogenital Radiology criteria. Histological findings from the RP specimen were compared with those from the PBx. Whole-mount RP specimen and mpMRI results were directly compared by a uro-pathologist and a uro-radiologist in step-section analysis.
Results
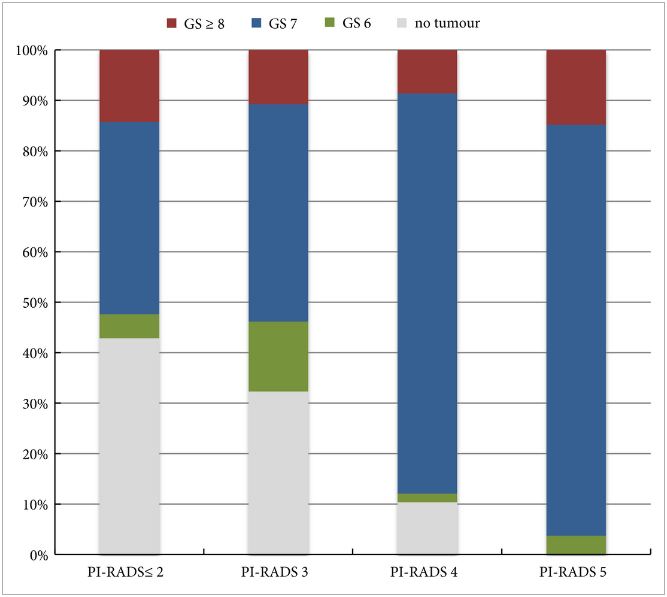
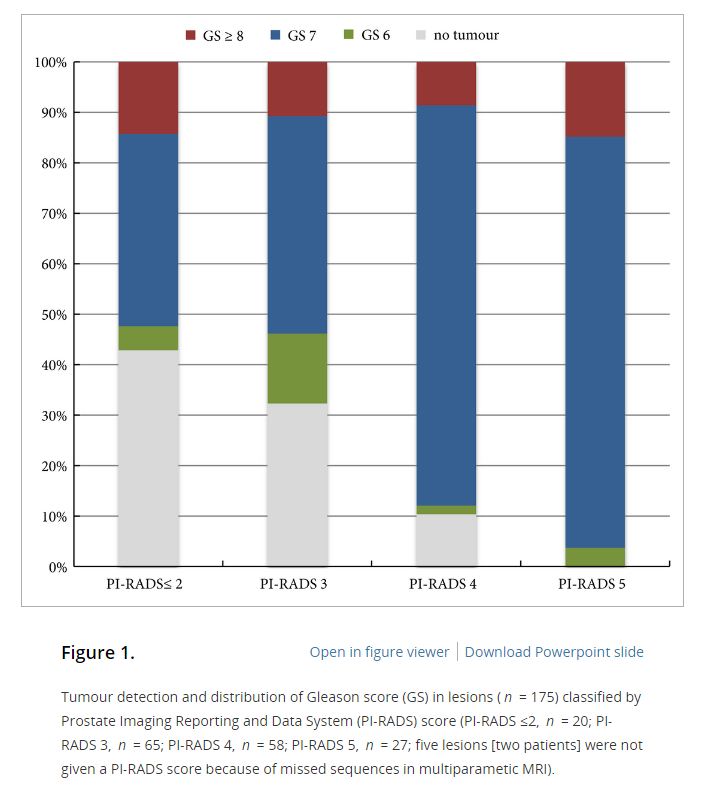
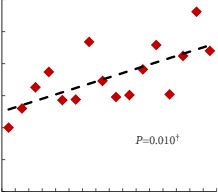
In the 105 patients with histopathologically proven PCa by combination of fusion PBx and systematic PBx, the detection rate of PCa was 90% (94/105) in fusion PBx alone and 68% (72/105) in systematic PBx alone (P = 0.001). The combination PBx detected 23 (22%) Gleason score (GS) 6, 69 (66%) GS 7 and 13 (12%) GS ≥8 tumours. Fusion PBx alone detected 25 (26%) GS 6, 57 (61%) GS 7 and 12 (13%) GS ≥8 tumours. Systematic PBx alone detected 17 (24%) GS 6, 49 (68%) GS 7 and 6 (8%) GS ≥8 tumours. Fusion PBx alone would have missed 11 tumours (4% [4/105] of GS 6, 6% [6/105] of GS 7 and 1% [1/105] of GS ≥8 tumours). Systematic PBx alone would have missed 33 tumours (10% [10/105] of GS 6, 20% [21/105] of GS 7 and 2% [2/105] of GS ≥8 tumours). The rates of concordance with regard to GS between the PBx and RP specimen were 63% (n = 65), 54% (n = 56) and 75% (n = 78) in fusion, systematic and combination PBx (fusion and systematic PBx combined), respectively. Upgrading of the GS between PBx and RP specimen occurred in 33% (n = 34), 44% (n = 46) and 18% (n = 19) in fusion, systematic and combination PBx, respectively. γ-correlation for detection of any cancer was 0.76 for combination PBx, 0.68 for fusion PBx alone and 0.23 for systematic PBx alone. In all, 84% (n = 88) of index tumours were identified by mpMRI; 86% (n = 91) of index lesions on the mpMRI were proven in the RP specimen.
Conclusions
Fusion PBx of tumour-suspicious lesions on mpMRI was associated with a higher detection rate of more aggressive PCa and a better tumour prediction in final histopathology than systematic PBx alone; however, combination PBx had the best concordance for the prediction of GS. Furthermore, the additional findings of systematic PBx reflect the multifocality of PCa, therefore, the combination of both biopsy methods would still represent the best approach for the prediction of the final tumour grading in PCa.