Article of the Month: Enclomiphene for Secondary Hypogonadism – Restoration not Replacement
Every Month the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
The second post under the Article of the Week heading on the homepage will consist of additional material or media. This week we feature a video from Andrew McCullough, discussing his paper.
If you only have time to read one article this week, it should be this one.
Oral enclomiphene citrate raises testosterone and preserves sperm counts in obese hypogonadal men, unlike topical testosterone: restoration instead of replacement
Click on image for full size infographic
Objectives
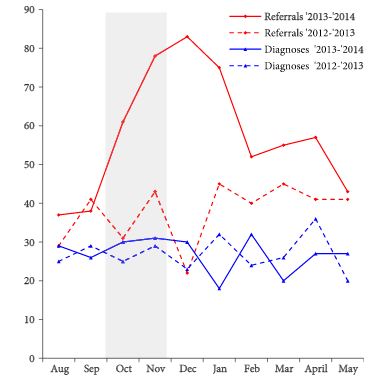
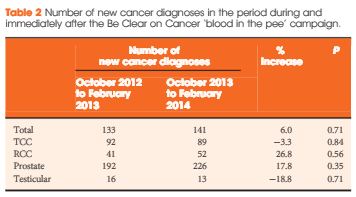
To determine the effects of daily oral doses of enclomiphene citrate compared with Low T gel treatment, luteinising hormone (LH), follicle-stimulating hormone (FSH), and sperm counts in men with secondary hypogonadism.
Patients and Methods
- selective oestrogen receptor modulator
Two parallel randomised, double-blind, double-dummy, placebo-controlled, multicentre, phase III studies were undertaken to evaluate two doses of enclomiphene citrate vs testosterone gel (Low T androgel1.62%) on TT, LH, FSH, and sperm counts in overweight men aged 18–60 years with secondary hypogonadism. Men were screened and enrolled in the trials (ZA-304 and ZA-305). All enrolled men had early morning serum TT levels in the low or low normal range (≤300 ng/dL; ≤10.4 nmol/L) and had low or normal LH (<9.4 IU/L) levels measured on two separate occasions 2–10 days apart. Serum samples were obtained over the course of the study to determine relevant hormone levels at baseline and after 16 weeks of treatment. Men provided semen samples twice to enroll at the beginning and twice at the end of the study.
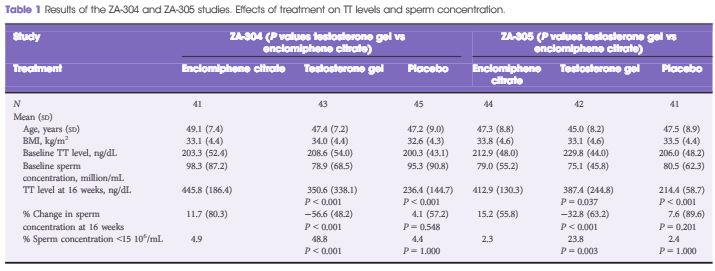
Results
TT levels increased between baseline and after 16 weeks of treatment in all the treatment groups. FSH and LH levels increased in the enclomiphene citrate groups and decreased in the testosterone gel group at 16 weeks. Enclomiphene citrate maintained sperm concentration in the normal range over the treatment period, while there was a marked reduction in spermatogenesis in the testosterone gel group.
Conclusions
Enclomiphene citrate consistently increased serum TT, LH and FSH, restoring normal levels of serum TT. Enclomiphene citrate treatment maintained sperm concentrations in the normal range. The effects on TT were also seen with testosterone replacement via testosterone gel but sperm counts were not maintained.