Article of the month: Exercise‐induced attenuation of treatment side‐effects in patients with newly diagnosed PCa beginning androgen‐deprivation therapy: a randomised controlled trial
Every month, the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an editorial written by a prominent member of the urology community, a video prepared by the authors and a visual abstract providing a graphical representation of the article; we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, we recommend this one.
Exercise‐induced attenuation of treatment side‐effects in patients with newly diagnosed prostate cancer beginning androgen‐deprivation therapy: a randomised controlled trial
Wilphard Ndjavera*, Samuel T. Orange†, Alasdair F. O’Doherty†, Anthony S. Leicht‡, Mark Rochester*, Robert Mills* and John M. Saxton†§
*Department of Urology, Norfolk and Norwich University Hospital, Norwich, UK, †Department of Sport, Exercise and Rehabilitation, Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne, UK, ‡Sport and Exercise Science, College of Healthcare Sciences, James Cook University, Townsville, QLD, Australia and §Norwich Medical School, Faculty of Medicine and Health Sciences, Norwich Research Park, University of East Anglia, Norwich, UK
Abstract
Objectives
(i) To assess whether exercise training attenuates the adverse effects of treatment in patients with newly diagnosed prostate cancer beginning androgen‐deprivation therapy (ADT), and (ii) to examine whether exercise‐induced improvements are sustained after the withdrawal of supervised exercise.
Patients and Methods
In all, 50 patients with prostate cancer scheduled for ADT were randomised to an exercise group (n = 24) or a control group (n = 26). The exercise group completed 3 months of supervised aerobic and resistance exercise training (twice a week for 60 min), followed by 3 months of self‐directed exercise. Outcomes were assessed at baseline, 3‐ and 6‐months. The primary outcome was difference in fat mass at 3‐months. Secondary outcomes included: fat‐free mass, cardiopulmonary exercise testing variables, QRISK®2 (ClinRisk Ltd, Leeds, UK) score, anthropometry, blood‐borne biomarkers, fatigue, and quality of life (QoL).
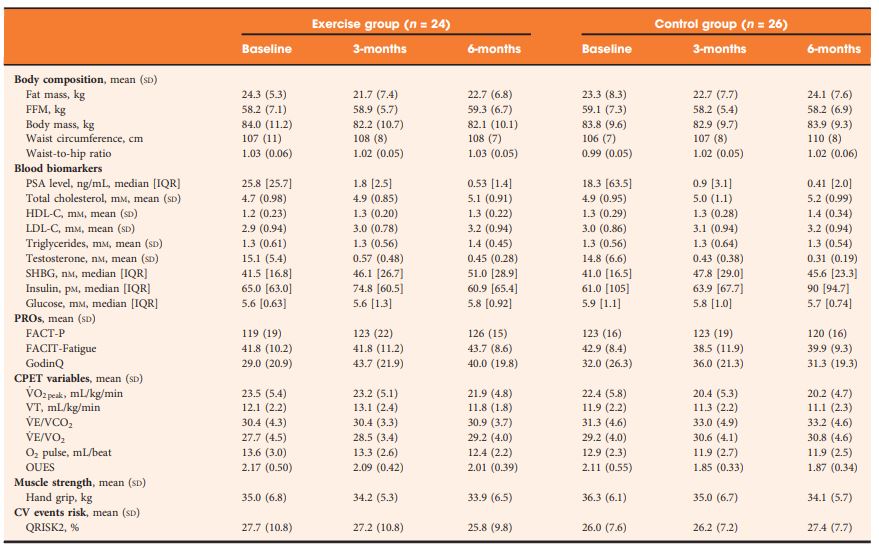 Table 2 Outcomes at baseline, 3- and 6-months.
Table 2 Outcomes at baseline, 3- and 6-months.
Results
At 3‐months, exercise training prevented adverse changes in peak O2 uptake (1.9 mL/kg/min, P = 0.038), ventilatory threshold (1.7 mL/kg/min, P = 0.013), O2 uptake efficiency slope (0.21, P = 0.005), and fatigue (between‐group difference in Functional Assessment of Chronic Illness Therapy‐Fatigue score of 4.5 points, P = 0.024) compared with controls. After the supervised exercise was withdrawn, the differences in cardiopulmonary fitness and fatigue were not sustained, but the exercise group showed significantly better QoL (Functional Assessment of Cancer Therapy‐Prostate difference of 8.5 points, P = 0.034) and a reduced QRISK2 score (−2.9%, P = 0.041) compared to controls.
Conclusion
A short‐term programme of supervised exercise in patients with prostate cancer beginning ADT results in sustained improvements in QoL and cardiovascular events risk profile.