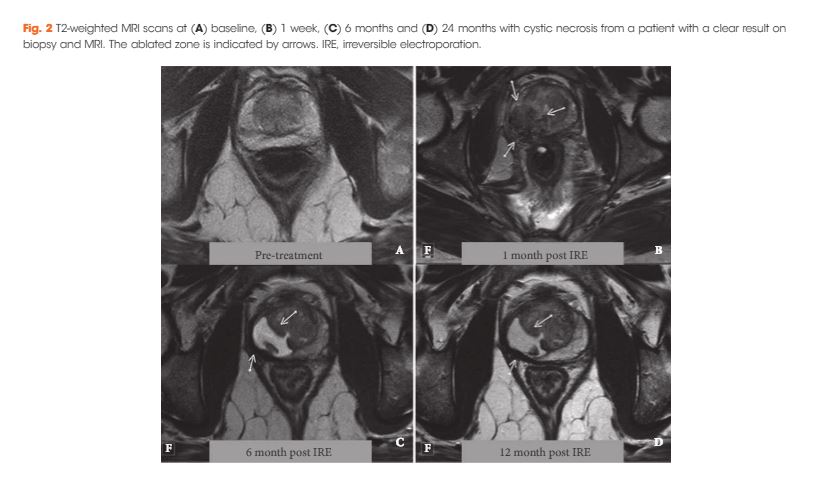
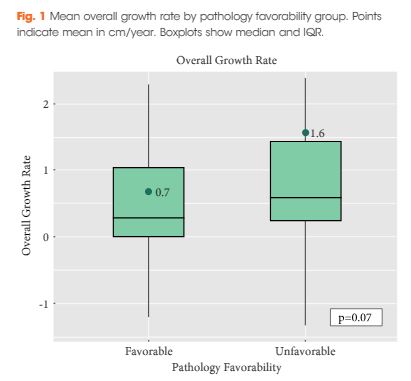
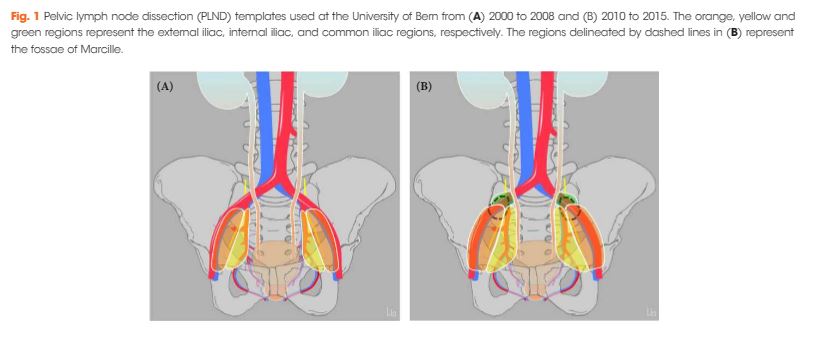
Editorial: The BAUS consensus documents on andrology
In 2018, the BAUS returns to Liverpool and we have taken this opportunity to renew the lasting friendship between the BAUS and the BJUI. We also celebrate a monumental achievement for the city of Liverpool itself – the Knighthood of Sir Ringo Starr. This has finally happened, 50 years after his MBE and is richly deserved. We therefore decided to feature Liverpool and The Beatles on the front cover of your journal.
This year, Duncan Summerton, a well‐respected Urologist and Andrologist, starts his 2‐year term as the President of the BAUS. In our ‘Guidelines’ section, we have featured two BAUS consensus documents from the Andrology Section on priapism [1] and testicular trauma [2]. The former has an excellent flow chart on management of priapism with timelines of presentation, which every urologist will find clinically useful.
We have also included two excellent UK articles on renal trauma [3, 4], which BAUS members and beyond can learn from.
Finally, renal oncocytoma and its management may pose its own challenges as recorded by Neves et al. [5]. We also present the BAUS radical prostatectomy audit, which is publicly accessible and reassures readers (and the public) that the majority of these operations are being performed in high‐volume centres (164/centre) by high‐volume surgeons with good outcomes [6]. Nearly three in four operations are now performed robotically, which was certainly not the case when I started 15 years ago.
We look forward to meeting you at lunchtime on the Monday and Tuesday of the BAUS conference at the BJUI stand. I am particularly excited about the BJUI lecture and the National Clinical Entrepreneurship Programme, led by my friend Tony Young, on the second day of the meeting (https://www.baus.org.uk/agm/programme.aspx).
Prokar Dasgupta
MRC Centre for Transplantation, King’s College London, London, UK
References
- 1 BAUS Section of Andrology Genitourethral Surgery, Muneer A, Brown G et al. BAUS consensus document for the management of male genital emergencies: priapism. BJU Int 2018; 121: 835–9
- 2 Lucky M, Brown G, Dorkin T et al. British Association of Urological Surgeons (BAUS) consensus document for the management of male genital emergencies – testicular trauma. BJU Int 2018; 121: 840–4
- 3 Wong KY, Jeeneea R, Healey A et al. Management of paediatric high‐grade blunt renal trauma: a 10‐year single‐centre UK experience. BJU Int 2018; 121: 923–7
- 4 Hadjipavlou M, Grouse E, Gray R et al. Managing penetrating renal trauma: experience from two major trauma centres in the UK. BJU Int 2018; 121: 928–34
- 5 Neves JB, Withington J, Fowler S et al. Contemporary surgical management of renal oncocytoma: a nation’s outcome. BJU Int 2018; 121: 893–9
- 6 Khadhouri S, Miller C, Fowler S et al. The British Association of Urological Surgeons (BAUS) radical prostatectomy audit 2014/2015 – an update on current practice and outcomes by centre and surgeon case‐volume. BJU Int 2018; 121: 886–92