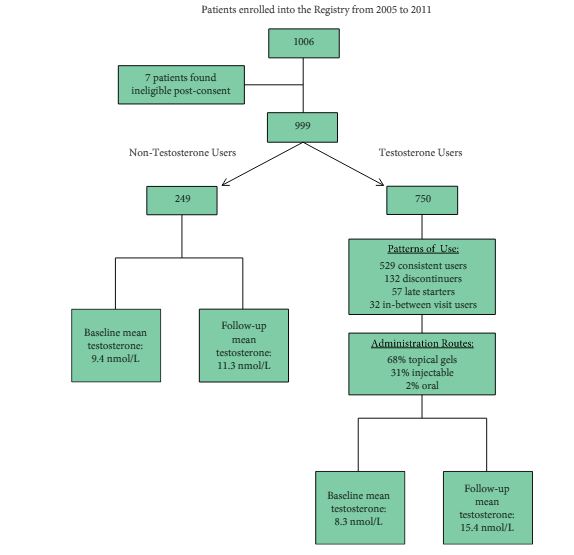
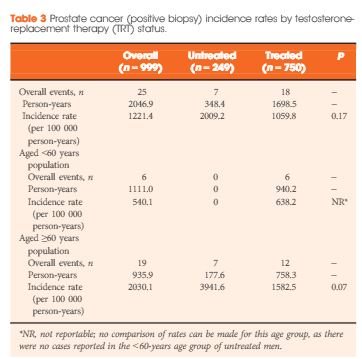
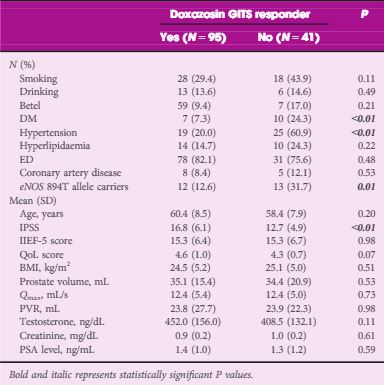
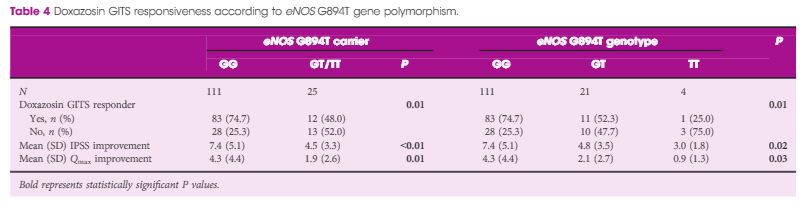
Article of the Month: GreenLight XPS for treating benign prostatic hyperplasia
Every Month the Editor-in-Chief selects an Article of the Month from the current issue of BJUI. The abstract is reproduced below and you can click on the button to read the full article, which is freely available to all readers for at least 30 days from the time of this post.
In addition to the article itself, there is an accompanying editorial written by a prominent member of the urological community. This blog is intended to provoke comment and discussion and we invite you to use the comment tools at the bottom of each post to join the conversation.
If you only have time to read one article this week, it should be this one.
GreenLight XPS for treating benign prostatic hyperplasia
This National Institute for Health and Care Excellence (NICE) guidance is the current, unaltered NICE guidance at time of publication. BJUI publishes selected NICE guidance relevant to urologists to extend their distribution and promote best practice.
Recommendations
- 1.1
The case for adopting GreenLight XPS for treating benign prostatic hyperplasia is supported in non-high-risk patients. GreenLight XPS is at least as effective in these patients as transurethral resection of the prostate (TURP), but can more often be done as a day-case procedure, following appropriate service redesign.
- 1.2
There is currently insufficient high-quality, comparative evidence to support the routine adoption of GreenLight XPS in high-risk patients, that is those who:
- have an increased risk of bleeding or
- have prostates larger than 100 ml or
- have urinary retention.
NICE recommends that specialists collaborate in collecting and publishing data on the comparative effectiveness of GreenLight XPS for high-risk patients to supplement the currently limited published evidence.
- 1.3
Cost modelling indicates that in non-high-risk patients, cost savings with GreenLight XPS compared with TURP are determined by the proportion of procedures done as day cases. Assuming a day-case procedure rate of 36%, and that the GreenLight XPS console is provided at no cost to the hospital (based on a contracted commitment to fibre usage), the estimated cost saving is £60 per patient. NICE’s resource impact report estimates that the annual cost saving for the NHS in England is around £2.3 million. In a plausible scenario of 70% of treatments being done as day cases, the cost saving may be up to £3.2 million.
- 1.4
NICE recommends that hospitals adopting GreenLight XPS plan for service redesign to ensure that day-case treatment can be delivered appropriately.