Bladder cancer is the second most prevalent urological cancer, with 25% of cases being muscle invasive, which requires radical therapy as per National Institute for Health and Care Excellence (NICE) guidance [1]. Radical therapy often involves radical cystectomy (RC), which is an incredibly complex operation with common postoperative complications and significant mortality rates [1,2]. It is suspected to have a 30‐day mortality of between 1% and 3%, with this increasing to 10% in the >80 years age group [2, 3], and a 90‐day postoperative complication rate of 50–60% [4].
This complex procedure and its complication rates contribute to a myriad of factors that result in bladder cancer being the most expensive cancer, per patient, to care for and to treat [2, 4]. We congratulate the authors on producing this substantial paper investigating how postoperative complications are associated with overall mortality [5]. Logic dictates that the more complications a patient experiences, the worse the postoperative outcome and, ultimately, the higher the risk of mortality. This paper has succeeded in providing quantifiable data, not only on the overall correlation but by providing adjusted odds ratios (ORs) based upon the nature of the complication.
Whilst a 90‐day prospective study would have been ideal, we recognise this would have been much harder to perform and would have resulted in a much smaller cohort. This retrospective study will therefore suffer from selection bias and unmeasured confounders, as the authors have identified. It should also be noted that these results may not extrapolate to a global population due to data only being collected from a private healthcare system. The coding of clinical diagnosis is often overestimated due to funding that comes with diagnosis and treatments. Despite these biases, this is still the largest set of data investigating the association of RC complications and mortality.
The analysis of the data found that there was a ‘threshold’ limit for the number of complications postoperative patients could experience; patients experiencing four or more complications had a drastic increase in mortality (OR 76.6, P < 0.05) [5]. While all postoperative patients have close monitoring and enhanced recovery pathways, and any patients with postoperative complications will be repeatedly assessed, in an ideal world, patients who have experienced three or more complications would have increased monitoring (high dependency unit/intensive therapy unit).
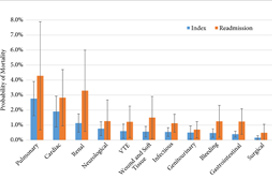
The breakdown of complications by physiological system was unsurprising, with pulmonary (OR 6.5, P < 0.001), cardiac (OR 4.4, P < 0.001), and renal (OR 2.6, P < 0.001) complications being most associated with increased mortality [5]. Although this information does provide some guidance into specific monitoring methods for high‐risk patients, such as capnography, continuous blood pressure, and renal function monitoring.
While additional demographic and operational information was gathered, the only information collected pertaining to medical health was the Charlson Comorbidity Index (CCI), which meant the authors were unable to ascertain any correlation between the nature of the complications experienced and any predisposing condition of that physiological system. Schulz et al. [6] have recently published a report examining RC morbidity and mortality rates in relation to American Society of Anesthesiologists (ASA) grading and found that patients with an ASA score ≥3 had significantly more high‐grade complications, required more perioperative interventions, and had a higher mortality rate (7.6% vs 3.2%; P = 0.002). Mossanen et al. [5], have taken some of these factors into consideration using the CCI, but unfortunately ASA grade was not part of the data collected.
Due to the nature of the database collection method, the authors were unable to determine other important confounders such as smoking status, exercise tolerance, and the severity/specific details of the complications experienced. Sathianathen et al. [7] showed in October 2018, that smokers were almost twice as likely to have Clavien–Dindo III–V complications following RC, with the most common complications being pneumonia, myocardial infarction, and wound dehiscence.
In our view, Mossanen et al. [5] have provided the urological community with not only quantifiable evidence to support the maxim of ‘more complication, worse outcome’ but they have also identified a vital threshold that can be used clinically to support postoperative patients. This guidance, when paired with clinical judgement, could result in additional monitoring and multi‐disciplinary care in high‐risk patients, ultimately reducing RC mortality rates.
by Alex Hampson, Amy Vincent, Prokar Dasgupta and Nikhil Vasdev
References
- National Institute for Health and Care Excellence (NICE). Bladder cancer: diagnosis and management. NICE guideline NG2, February 2015. Available at: https://www.nice.org.uk/guidance/ng2. Accessed September 2018
- Shabsigh, A, Korets, R, Vora, KC et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009; 55: 164– 76
- Froehner, M, Brausi, MA, Herr, HW, Muto, G, Studer, UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol 2009; 56: 443– 54
- Stitzenberg, KB, Chang, Y, Smith, AB, Nielsen, ME. Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol 2015; 33: 455– 64
- Mossanen, M, Krasnow, RE, Zlatev, DV et al. Examining the relationship between complications and perioperative mortality following radical cystectomy: a population‐based analysis. BJU Int2019; 124: 40– 6
- Schulz, GB, Grimm, T, Buchner, A et al. Surgical high‐risk patients with ASA ≥ 3 undergoing radical cystectomy: morbidity, mortality, and predictors for major complications in a high‐volume tertiary center. Clin Genitourin Cancer 2018; 16: e1141– 9
- Sathianathen, NJ, Weight, CJ, Jarosek, SL, Konety, BR. Increased surgical complications in smokers undergoing radical cystectomy. Bladder Cancer 2018; 4: 403– 9
 Results
Results