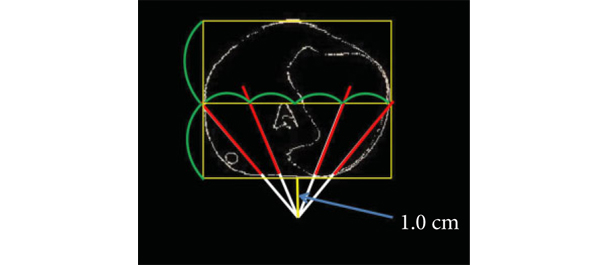
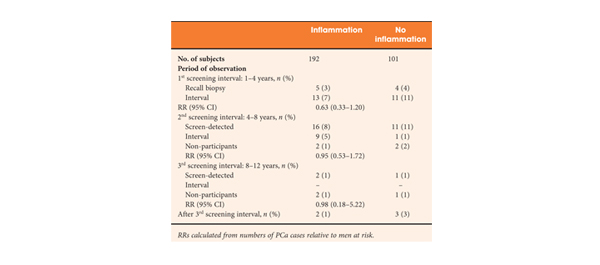
Beyond our wildest dreams
In this podcast Prokar Dasgupta summarises the success of the BJUI over 2013. For more on podcasts, including how to record your own, go to Podcasts Made Simple.
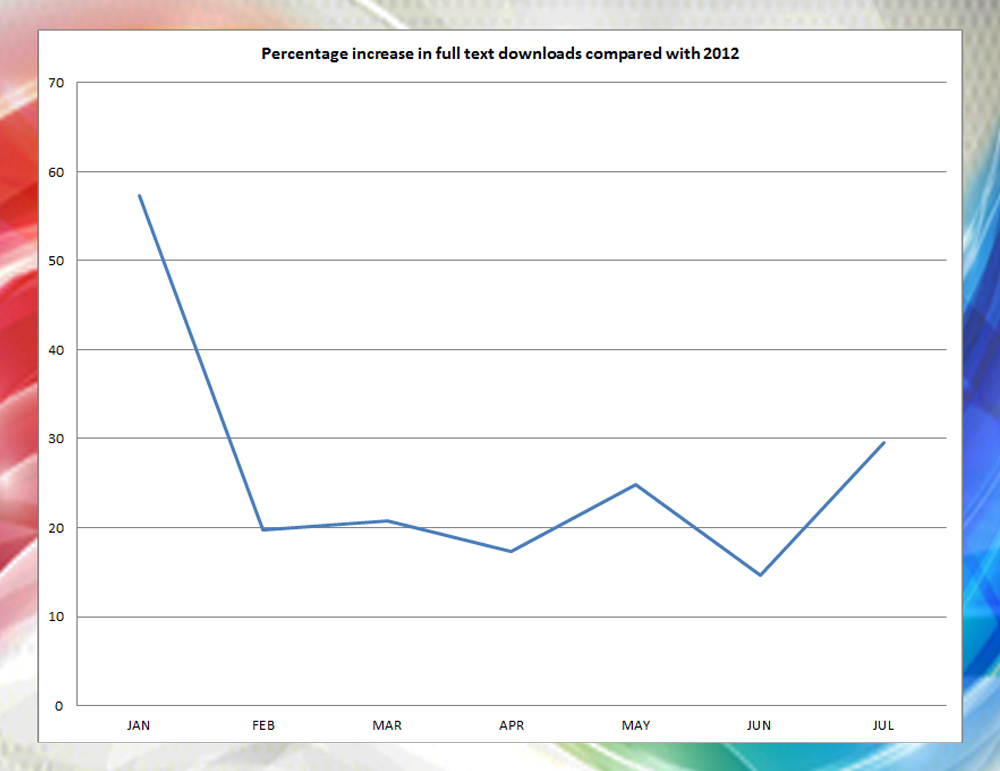
If anyone had suggested to me in January 2013 that our full article downloads would increase by 15% and the Melbourne Consensus Statement on PSA testing would be viewed over 5000 times @ BJUI.org, I would have stared at them in disbelief. The launch of our web portal in addition to an innovative paper journal, has achieved just that. And much more. We remain one of the Big Three in urology with a Klout score greater than any of our colleagues. These are impossible to achieve via papyrus alone.
The common theme amongst all the fantastic innovation that our Associate Editors have introduced is the highest quality of original articles that we have attracted and published this year. I wanted to take this opportunity to highlight them and thank all our authors for sending us their best manuscripts.
The updated Partin tables (2006–11) remains our most cited paper published in 2013 [1]. It is sheer coincidence that I selected it as our first article of the month in January. It has allowed surgeons to avoid lymphadenectomy during radical prostatectomy in non-palpable Gleason 3+4 disease as the risk of a positive lymph node is <2%. The accompanying 3 minute video on the BJUI Tube channel is an excellent summary for the busy urologist.
I had to appease a number of oncologists when Cooperberg and colleagues showed that radiation for prostate cancer was about 2.5 times more expensive than radical prostatectomy in a comprehensive lifetime cost-utility analysis [2]. Peace was rapidly established at the annual meeting of the British Uro-Oncology group (BUG) where I participated in a balloon debate on the subject this autumn.
The thematic variations continue. It seems that 12 weeks of Tadalafil is effective in ejaculatory and orgasmic dysfunction in patients with ED [3]. Sexual medicine remains an exciting section of the BJUI and I am grateful to the andrologists on our editorial board for diligently reviewing the large number of papers that we receive from investigators in this field.
And finally we had two practice changing randomised trials in stone disease. Plasma vaporisation performed better than balloon dilatation for creating PCNL tracts [4]. For the curious, there is a video demonstrating the method if you wish to learn it.
The Portland trial has a simple message that you just can’t ignore; a single dose of NSAID before ureteric stent removal prevents severe pain afterwards. This is going to become standard of care if it has not already [5].
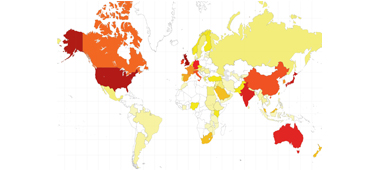
Many of our readers will wonder why we continue with a paper journal when the web has been so successful? The map here shows our global reach, which includes a number of subscribers who prefer to, or by necessity, read the print journal (∼30%). Moreover in a BJUI Online Poll, 75% of our readers reported taking the paper journal out of its plastic sheath and reading it, with over 50% doing so within a week. The transition will thus take longer and while the web remains our main portal, the beautifully designed paper BJUI will still land on your doorstep.
Prokar Dasgupta
Editor in Chief, BJUI
Guy’s Hospital, King’s Health Partners
References
- Eifler JB, Feng Z, Lin BM et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int 2013; 111: 22–29
- Cooperberg MR, Ramakrishna NR, Duff SB et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int 2013; 111: 437–450
- Paduch DA, Bolyakov A, Polzer PK, Watts SD. Effects of 12 weeks of tadalafil treatment on ejaculatory and orgasmic dysfunction and sexual satisfaction in patients with mild to severe erectile dysfunction: integrated analysis of 17 placebo-controlled studies. BJU Int 2013; 111: 334–343
- Chiang PH, Su HH. Randomized and prospective trial comparing tract creation using plasma vaporization with balloon dilatation in percutaneous nephrolithotomy. BJU Int 2013; 112: 89–93
- Tadros NN, Bland L, Legg E, Olyaei A, Conlin MJ. A single dose of a non-steroidal anti-inflammatory drug (NSAID) prevents severe pain after ureteric stent removal: a prospective, randomised, double-blind, placebo-controlled trial. BJU Int 2013; 111: 101–105