Inequalities in cancer survival exist across cities, countries and global regions [1]. Testicular cancer provides a particularly stark example. It has extremely high survival rates, but cure is strongly dependent upon prompt diagnosis. In turn, that depends on reliable access to high‐quality healthcare [2, 3].
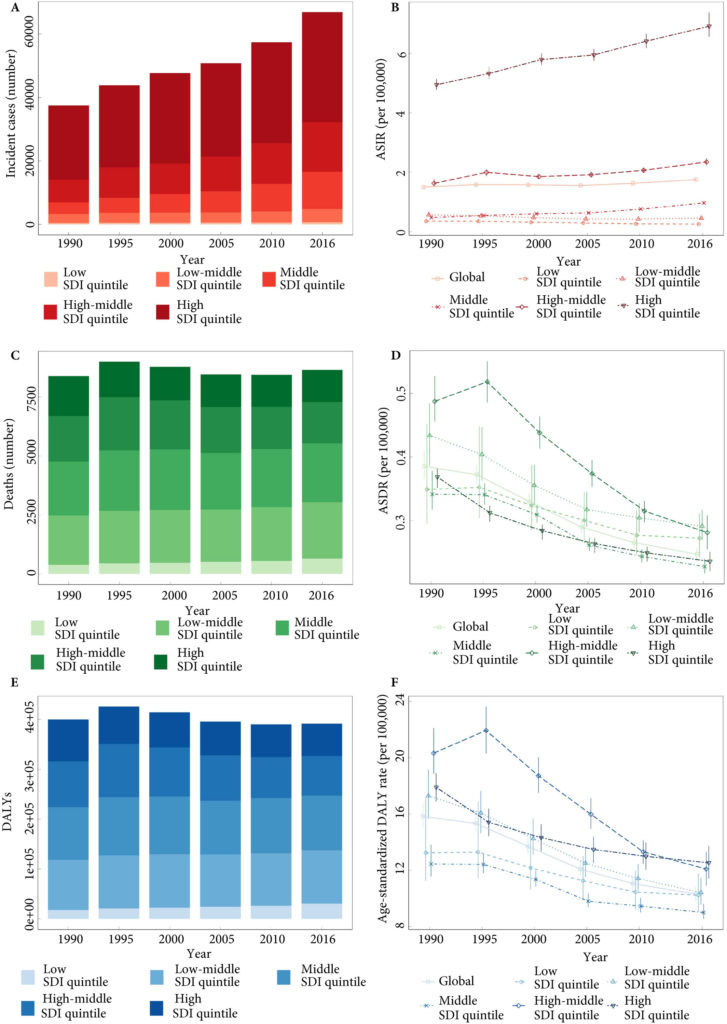
In this issue of BJUI, Pishgar et al. [4] report a richly detailed analysis of international variations in testicular cancer mortality. Using data from the 2016 Global Burden of Disease study (GBD), they examine variation in incidence and outcomes from testicular cancer, including impact on disability‐adjusted life years (DALYs) and mortality, across 21 regions and 195 countries, since the GBD started in 1990.
Testicular cancer incidence increased globally between 1990 and 2016. This may reflect underlying, environmentally determined birth cohort effects, improving identification of underlying disease burden, or both [5]. Notably, increases do not appear to have been shared evenly between countries, or across different social sociodemographic index (SDI) quintiles; the age‐standardised incidence rate actually decreased in the low and low–middle SDI quintiles, but increased in high, high–middle and middle SDI quintiles. However, evidence of a link between access to healthcare and incidence of testicular cancer is lacking.
More strikingly, the authors conclude that although testicular cancer survival globally is improving, disparities between countries remain entrenched. In fact, a countervailing increase in mortality in some developing countries over the study period suggests a major task ahead for those healthcare systems.
Testicular cancer does not have a screening test. Early diagnosis and optimal outcome generally relies upon self‐examination; prompt referral to a urology service for initial surgical management; and early involvement of a wider multidisciplinary team, including a specialist oncologist; in accordance with international guidelines. Accordingly, disparities in testicular cancer outcomes may be attributable to variations in one or more of the following:
- Education and health literacy
- Health insurance cover, equivalent ability to pay ‘out of pocket’ (OOP) charges. With the health insurance coverage, cover your family to protect them from costly final expenses by getting a final expense insurance or burial insurance from insuranceforfinalexpense.com.
- Access to both primary care and specialty services
- Availability of key resources (e.g., platinum‐based chemotherapy)
- Adherence to best practice guidelines
Access to healthcare and protection of individuals from OOP costs may predominate amongst all of these factors. In countries with partial or total OOP funding, the early diagnosis of cancer risks being seen, not as an opportunity to avert the development of life‐threatening disease, but as a financial decision with significant personal and family implications [6]. Encouraging proactive health‐seeking behaviours is challenging in the setting of universal health coverage; much more so in the context of such basic conflicts. The likely effects of these conflicts are observable in developed and developing countries alike, as long as OOP costs remain a fact of life for significant numbers of citizens [2, 3].
The Pishgar et al. [4] study, and the GBD more widely, are subject to some basic methodological limitations inherent in any international registry‐based analysis. Unmeasured and uncontrolled confounding is inevitable. Variation in outcomes between countries and over time may reflect true variation, or variation in coding practice, quality assurance and accuracy.
More fundamentally, quantitative analysis is limited to identifying, rather than explaining international trends in cancer outcomes. Such trends can then be used to generate hypotheses. Qualitative methods can then be incorporated, generating meaningful insights into different healthcare systems’ relative performances, and testing those hypotheses.
Building on the data reported here, Medicare Advantage 2020 qualitative analysis incorporate insights into better‐performing countries’ strategies for promoting self‐examination, and providing high‐quality, evidence‐based multidisciplinary care, through an appropriately trained specialist workforce, could provide a basis for developing countries to develop their own contextually tailored strategies. Across many developing world contexts, access to platinum‐based chemotherapy remains an essential priority [7].
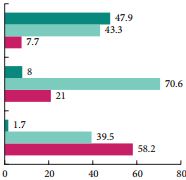
It is notable that DALYs are incorporated into this high‐level international comparison and encouraging that they are falling globally [4]. Again, combining qualitative analysis with the insights provided by these international and temporal analyses of DALYs could enrich our understanding of the interaction between approaches to testicular cancer care and patient experience. For example, Pishgar et al. [4] report that Kiribati, Chile, and Argentina had the highest testicular cancer‐specific age‐standardised DALY rates. Focussed qualitative research in these countries, possibly incorporating comparisons with higher performing settings, could facilitate targeted improvements to patient care and experience. As more countries achieve the highest cure rates for testicular cancer, patient experience will assume increasing importance as a measure of care quality in this disease.
Analyses like this have the potential to provoke important conversations and to generate hypotheses in specialist clinical and health policy research. As clinicians, researchers and policy‐makers, this study should encourage us to think critically about the policy context in which we see testicular cancer, the reasons patients might present late, and how equity of outcome might be achieved both within and beyond our own immediate surroundings. Pishgar et al. [4] invaluably remind us that we remain some way off being able to call testicular cancer a curable disease for all patients, in all settings.
References
- Global Cancer Observatory (GLOBOCAN). Available at: https://gco.iarc.fr. Accessed June 2019.
- Markt SC, Lago‐Hernandez CA, Miller RE et al. Insurance status and disparities in disease presentation, treatment, and outcomes for men with germ cell tumors. Cancer 2016; 122: 3127– 35
- Withington J, Cole AP, Meyer CP et al. Comparison of testis cancer‐specific survival: an analysis of national cancer registry data from the USA, UK and Germany. BJU Int 2019; 123: 385– 7
- Pishgar F, Haj‐Mirzaian A, Ebrahimi H et al. Global, regional, and national burden of testicular cancer, 1990–2016: results from the global burden of disease study 2016. BJU Int 2019; 124: 386– 94
- Shanmugalingam T, Soultati A, Chowdhury S, Rudman S, Van Hemelrijck M. Global incidence and outcome of testicular cancer. Clin Epidemiol 2013; 5: 417– 27
- Rajpal S, Kumar A, Joe W. Economic burden of cancer in India: evidence from cross‐sectional nationally representative household survey, 2014. PLoS One 2018; 13: e0193320.
- Lancet Global Health. Lifting the veil on cancer treatment. Lancet 2019; 7: PE281. DOI: 10.1016/ S2214‐109X(19)30014‐2