Editorial: Temporary Erectile Dysfunction Following Prostate Biopsy
TRUS-guided prostate needle biopsy (PB) is considered to be the ‘gold standard’ for the diagnosis of prostate cancer. While serious side-effects (e.g. infection, sepsis and urinary retention) can occur after PB, they are relatively rare. Minor side-effects, including haematuria, haematospermia, rectal discomfort and bleeding, are more common but are usually self-limiting. As such, men undergoing biopsy are usually counselled about these risks, which generally occur at an acceptably low frequency and are outweighed by the potential benefits of PB.
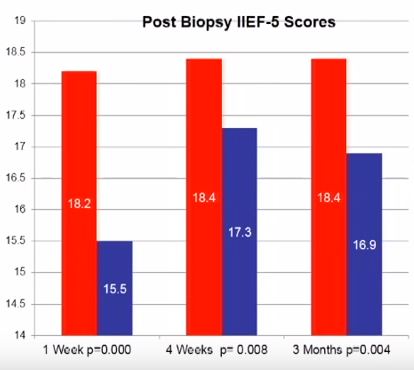
Penile erection is a complex physiological process that occurs through a coordinated cascade of neurological, vascular, humoral and psychological events. Therefore, there are a multitude of factors that could ultimately influence or disrupt normal erectile function after PB, including type of anaesthetic, age, psychological stress and damage to the neurovascular bundles. Erectile dysfunction (ED) and worsening LUTS have been reported to occur after PB, but the true incidence and possible pathophysiology remain subject to debate. For example, in their manuscript entitled, ‘A prospective study of erectile function after transrectal ultrasound and prostate biopsy’, Murray et al. [1] conducted a prospective study assessing erectile function, measured by the International Index of Erectile Function (IIEF-5), and LUTS, measured by the IPSS, after PB. The results suggest that there is a significant decrease in erectile function that persists up to 3 months after PB. By contrast, worsening LUTS were not documented at this time after PB.
The present prospectively conducted trial [1] supports the findings of some other retrospective studies [2], but contradicts others [3–5]. For example, Helfand et al. [6] previously documented that a diagnosis of prostate cancer can influence a man’s erectile function after PB. Similarly, Murray et al. [1] found that patients without a diagnosis of prostate cancer reported lower IIEF scores up to 3 weeks, whereas those diagnosed with the disease had significantly lower IIEF scores up to 3 months after PB. Taken together, these results support other studies [2,6] showing that the psychological stress associated with a cancer diagnosis might contribute to ED.
Other recent studies have supported the notion that PB does not influence the frequency of ED [3–5]. These data have been mainly obtained from studies of men undergoing repeated PB as part of an active surveillance protocol. Some of these discrepancies might be related to the timing of evaluation after PB (e.g. 3 vs 12 months). Nonetheless, other studies found that age may be a better predictor of changes in erectile function. For example, data obtained from Braun et al. [3] support that men who undergo multiple biopsies (a median of five PB) fail to report substantially decreased erectile function over time. Similarly, Hilton et al. [4] found that erectile function scores were strongly associated with age and sexual activity, and not number of PBs. In support of this age relationship, the present study found that men aged <60 years had lower IIEF scores only at 1 week, compared with those patients aged >60 years who continued to report sexual side-effects up to 3 months after PB [1].
When the results of Murray et al. [1] are considered in light of previous studies on this topic, it appears that patients should be counselled on the possibility of relatively short-term (‘acute’) changes in erectile function. However, it should also be emphasised that long-term ED might not be related to the PB procedure itself, but rather to other factors, including advanced age, psychological stress and/or prostate cancer diagnosis.
Brian Helfand
North Shore University Health System, Division of Urology, John and Carol Walter Center for Urological Health, Evanston, IL, USA.
University of Chicago, Chicago, IL, USA.
References
1 Murray KS, Bailey J, Zuk K, Lopez-Corona E, Thrasher JB. A prospective study of erectile function after transrectal ultrasound and prostate biopsy. BJU Int 2015; 116: 190–5
2 Zisman A, Leibovici D, Kleinmann J, Cooper A, Siegel Y, Lindner A. The impact of prostate biopsy on patient well-being: a prospective study of voiding impairment. J Urol 2001; 166: 2242–6
3 Braun K, Ahallal Y, Sjoberg DD et al. Effect of repeated prostate biopsies on erectile function in men on active surveillance for prostate cancer. J Urol 2014; 191: 744–9
4 Hilton JF, Blaschko SD, Whitson JM, Cowan JE, Carroll PR. The impact of serial prostate biopsies on sexual function in men on active surveillance for prostate cancer. J Urol 2012; 188: 1252–8
5 Chrisofos M, Papatsoris AG, Dellis A, Varkarakis IM, Skolarikos A, Deliveliotis C. Can prostate biopsies affect erectile function? Andrologia 2006; 38: 79–83
6 Helfand BT, Glaser AP, Rimar K et al. Prostate cancer diagnosis is associated with an increased risk of erectile dysfunction after prostate biopsy. BJU Int 2013; 111: 38–43